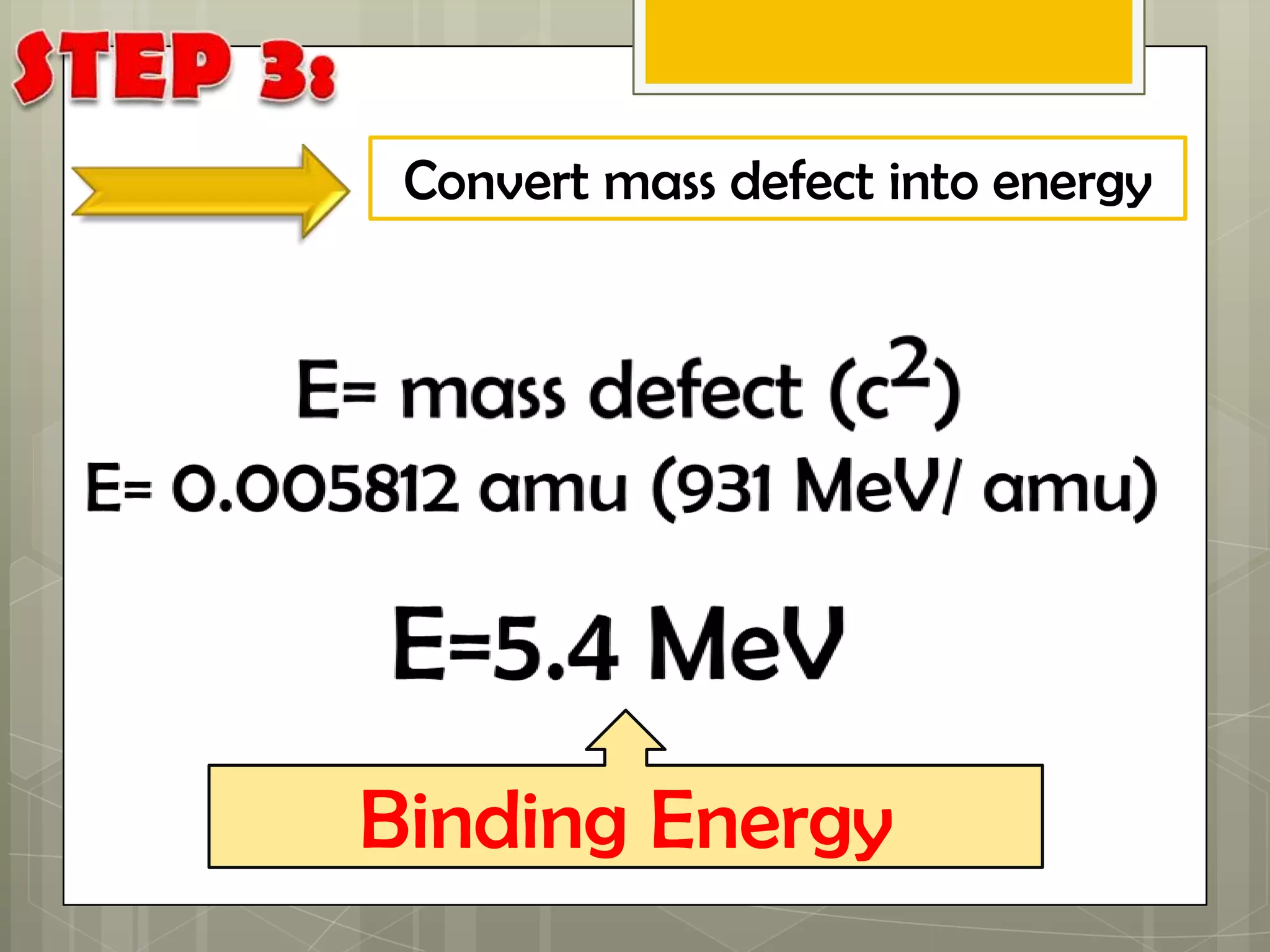
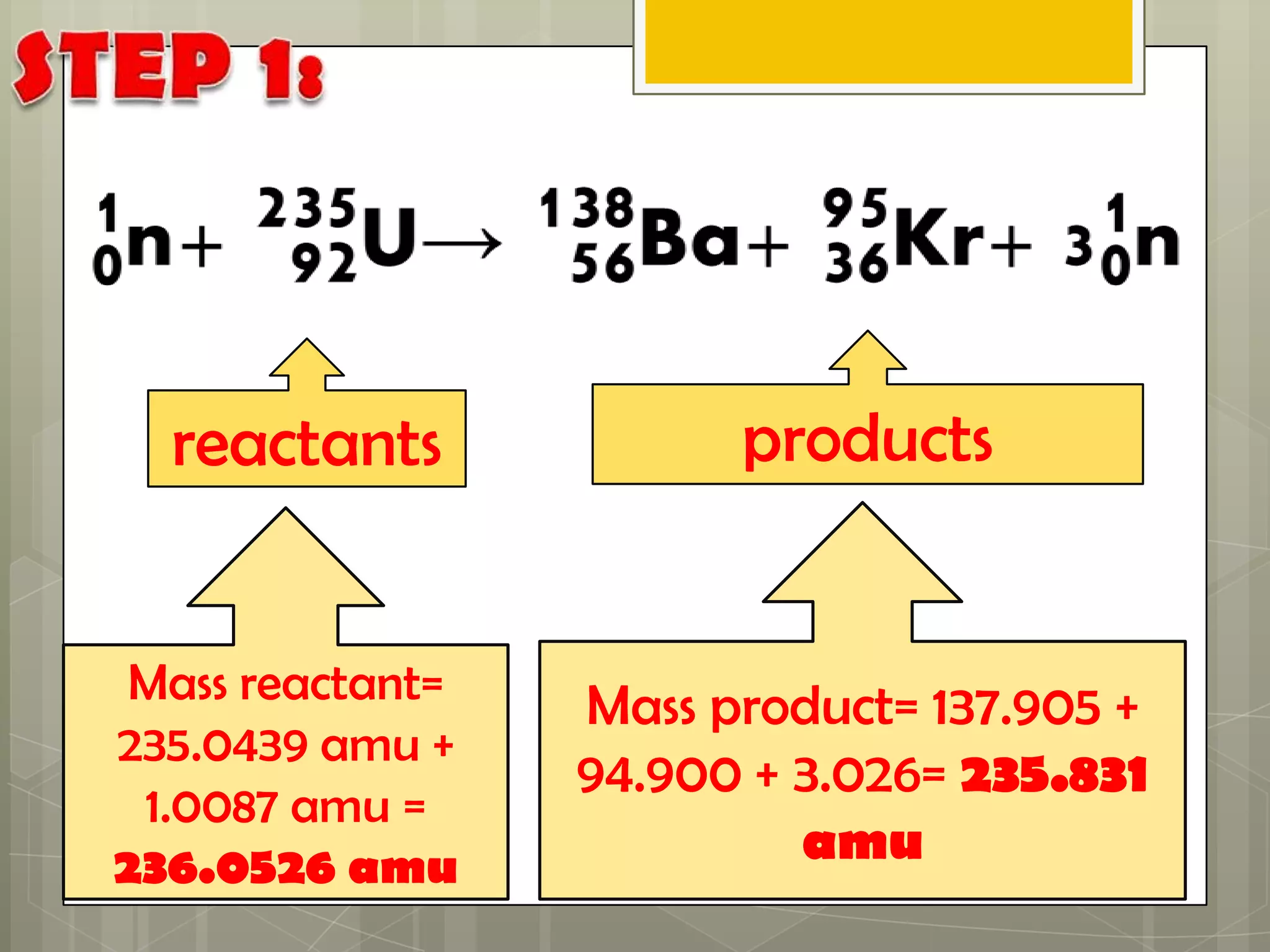
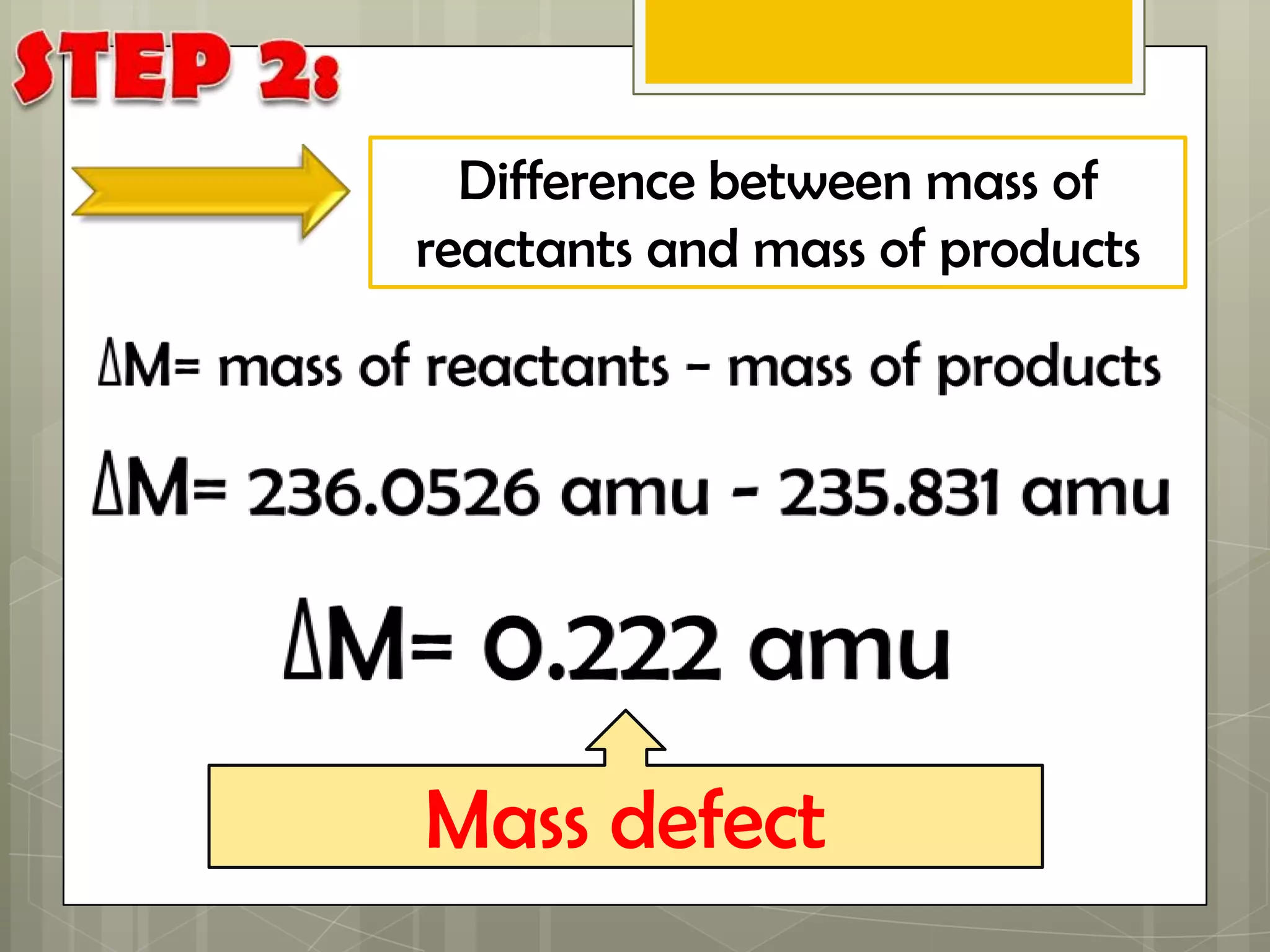
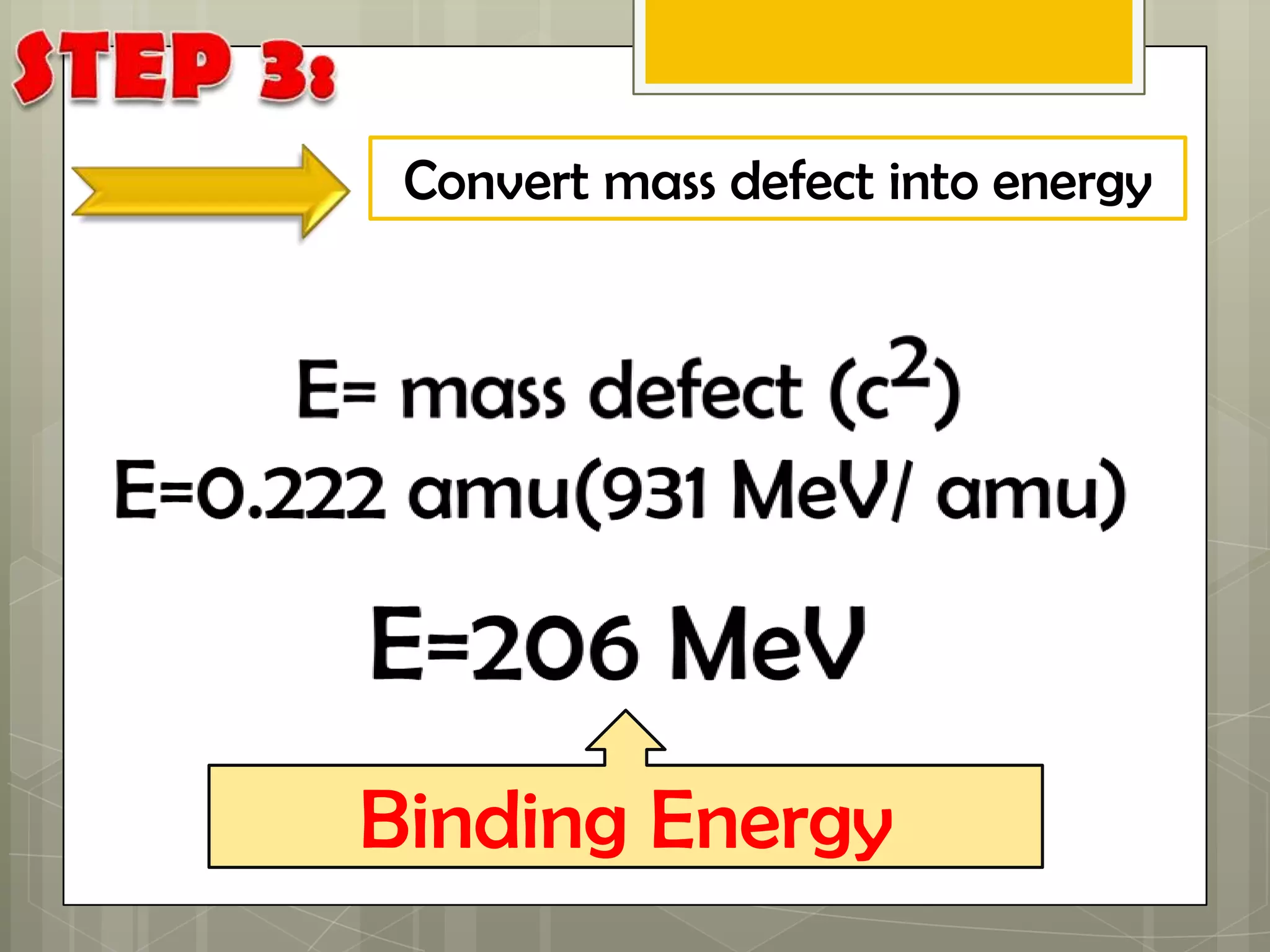
Here is a summary of the key points in 1⁄2 page:
The Bataan Nuclear Power Plant (BNPP) was built in the Philippines in the 1970s but has never been operational. There were several issues that prevented its operation:
1. Safety concerns - The plant was built during the 1970s before stricter safety standards were established after the Three Mile Island accident in 1979. International experts raised doubts about its ability to withstand earthquakes and other natural disasters common in the Philippines.
2. High costs of operation - Estimates showed the plant would incur huge operating costs, especially for maintenance and purchasing nuclear fuel. These costs were deemed too high for the Philippine government and electricity consumers.
3.