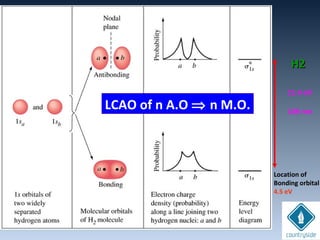
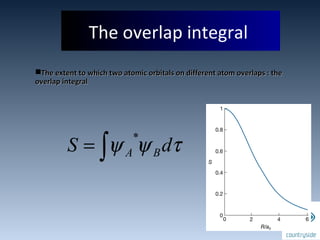
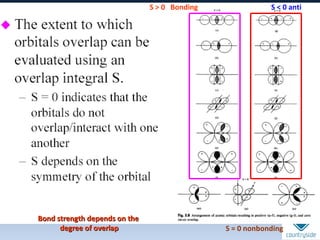
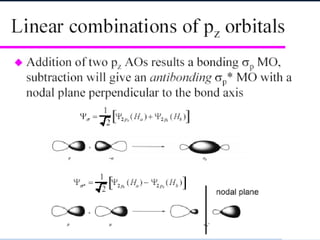
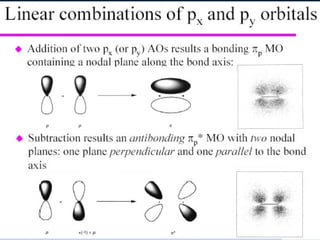
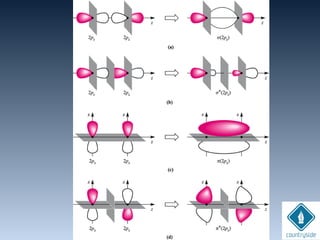
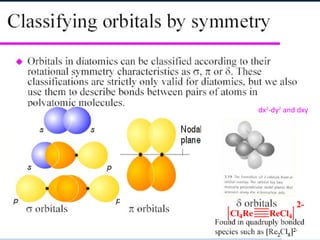
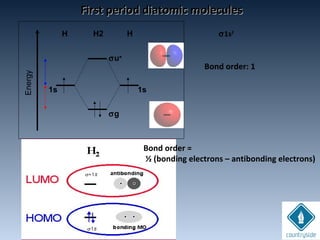
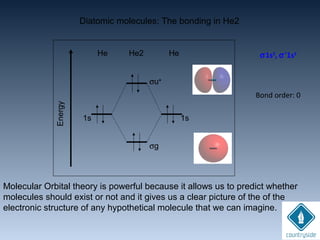
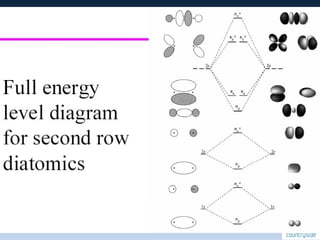
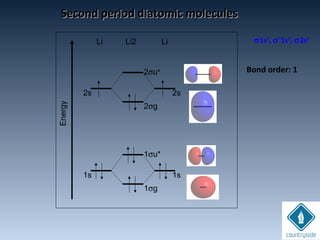
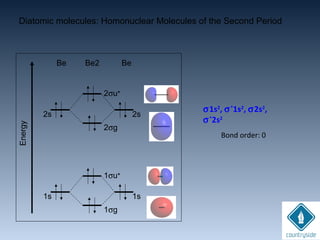
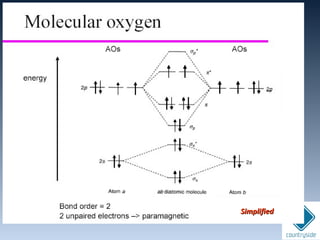
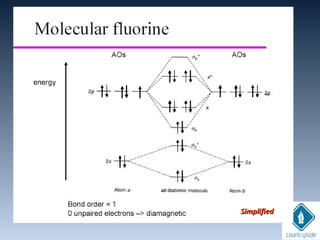
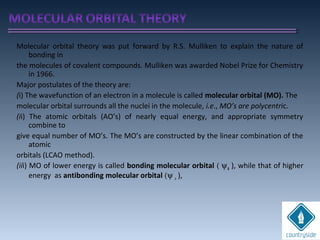
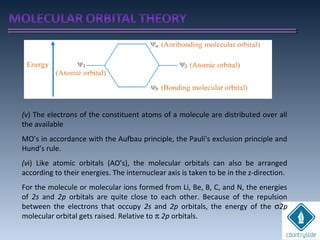
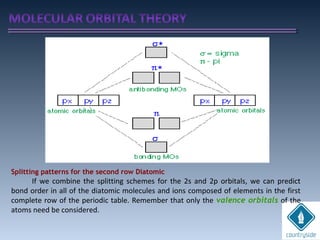
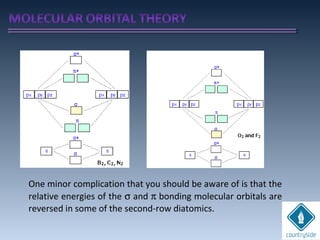
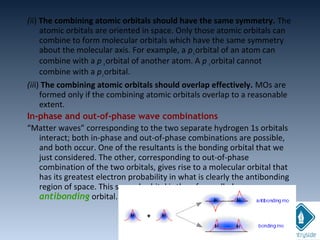
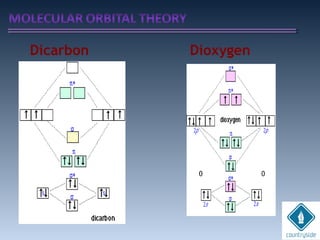
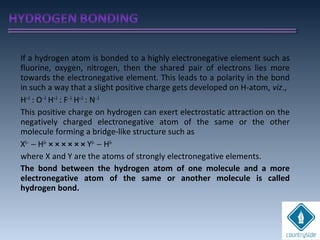
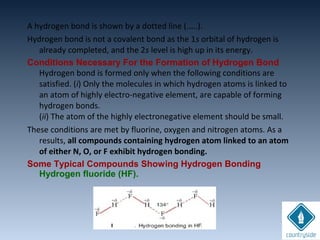
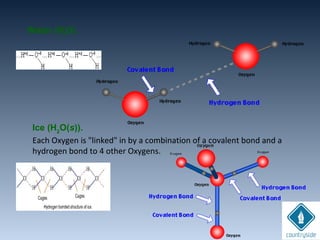
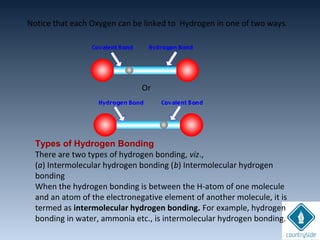
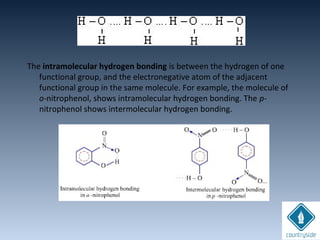
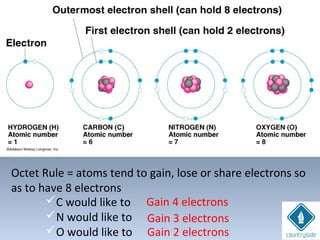
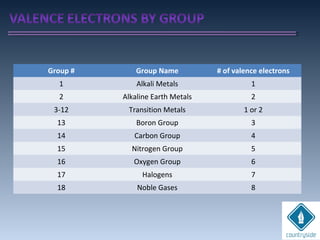
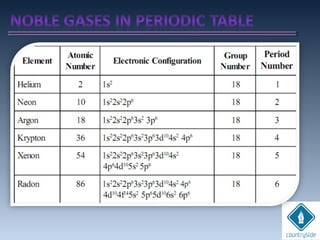
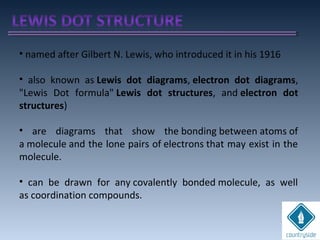
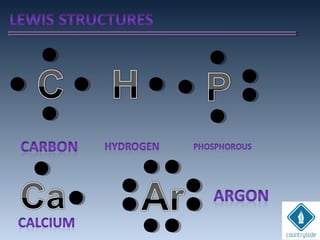
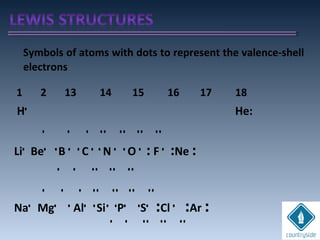
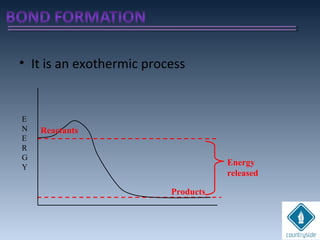
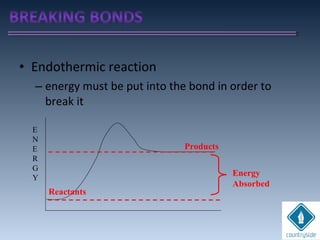
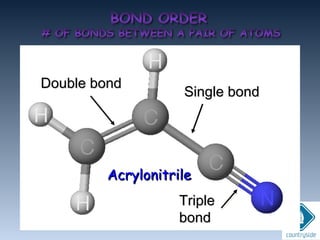
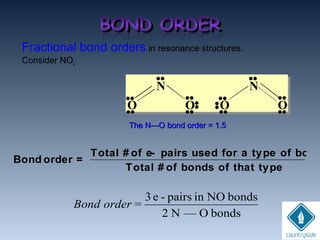
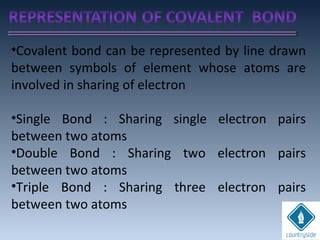
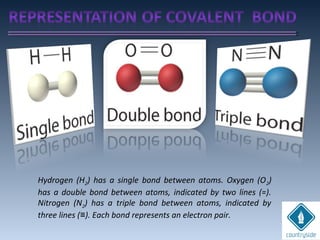
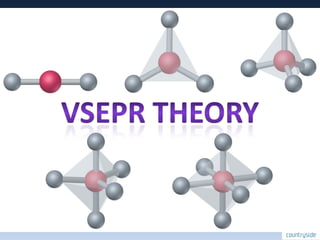
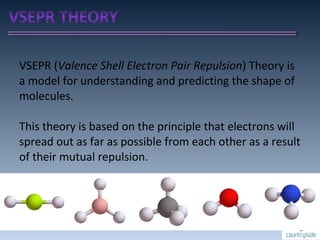
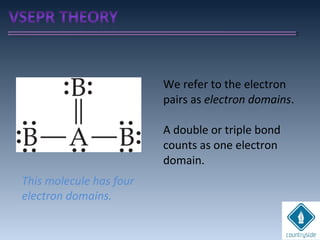
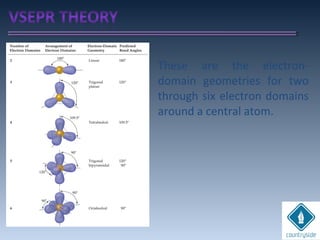
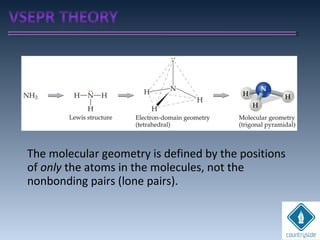
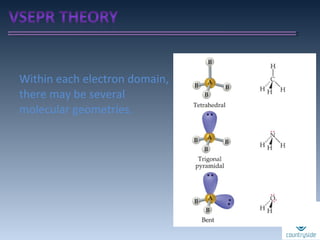
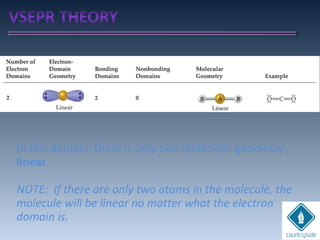
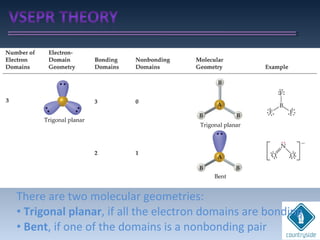
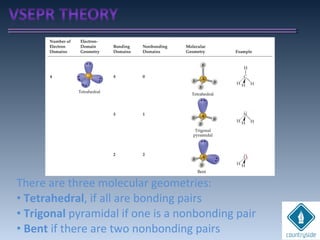
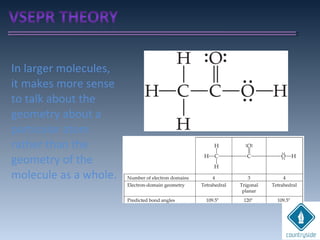
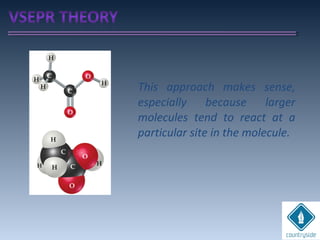
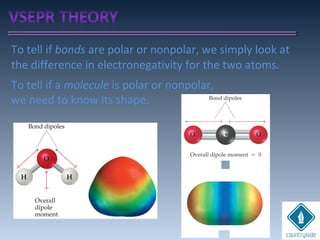
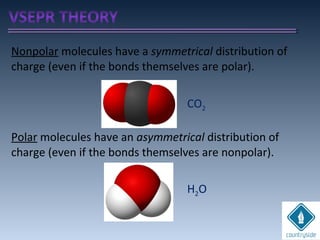
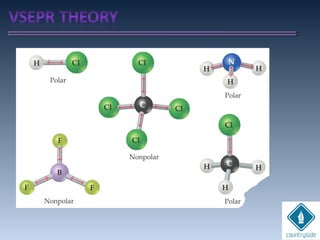
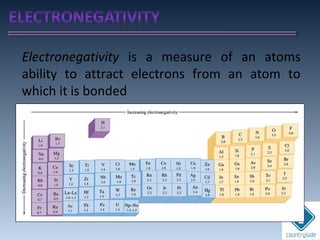
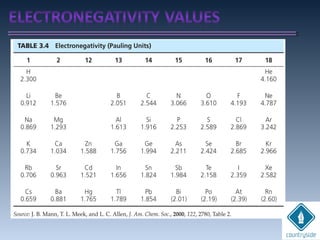
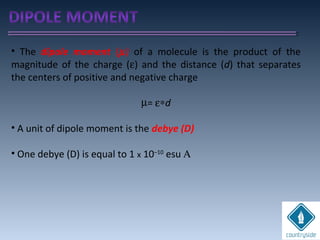
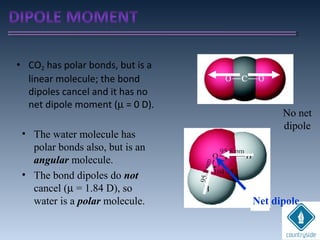
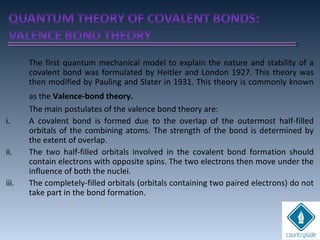
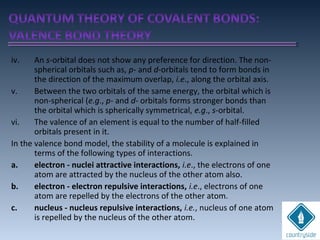
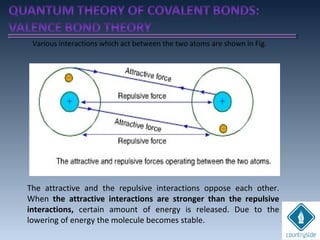
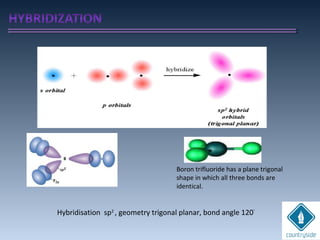
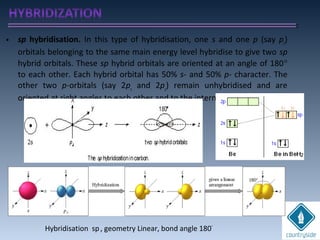
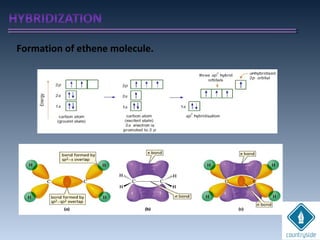
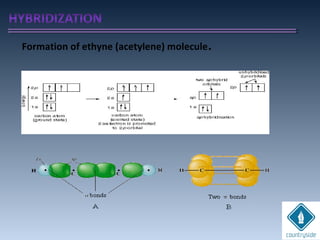
The document provides an overview of chemical bonding concepts, including valence electrons, types of bonds (covalent, ionic, and hydrogen bonds), and related theories like hybridization, VSEPR, and resonance. It discusses the significance of bond dipole moments, electronegativity, bond lengths, and the octet rule in determining the stability and reactivity of atoms. Additionally, it outlines the Born-Haber cycle, the nature of chemical compounds, and various bonding structures and properties associated with different elements.













![Doesn’t allow for
• H, He or Li
[stable with 2 e-
in their outer shells] -
Duet Rule
• Transition elements - 18 electron rule
• BF3 which only has 6 e-
in its outer shell](https://image.slidesharecdn.com/chemicalbondingandmolecularstructure-130720150057-phpapp02/85/Chemical-bonding-and-molecular-structure-17-320.jpg)

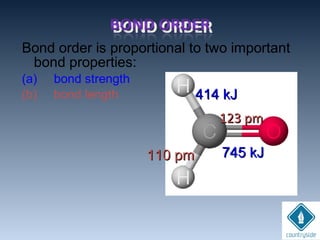



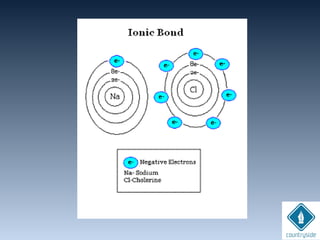
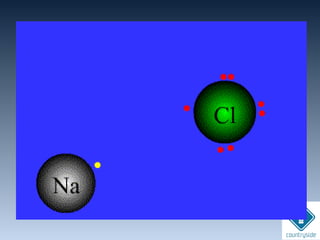
![• Always formed between metals and non-
metals
[METALS ]+
[NON-METALS ]
-
Lost e-
Gained e-](https://image.slidesharecdn.com/chemicalbondingandmolecularstructure-130720150057-phpapp02/85/Chemical-bonding-and-molecular-structure-38-320.jpg)










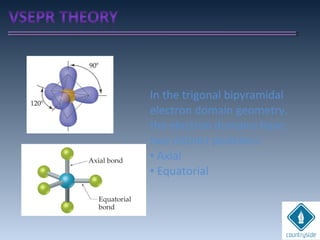
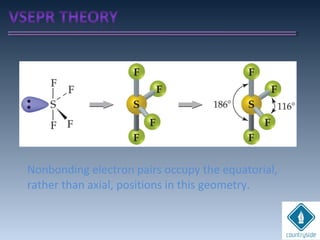
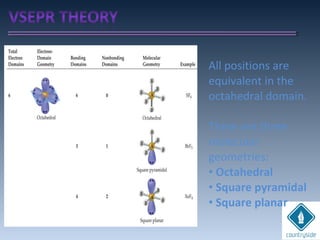
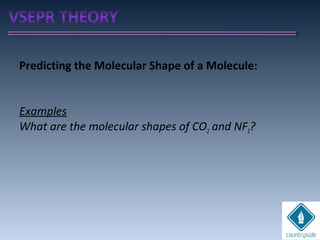




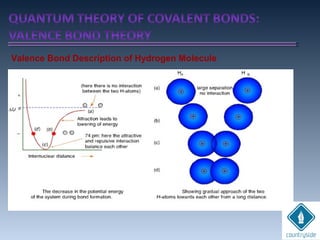







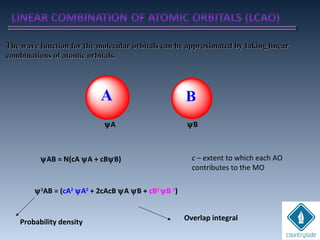
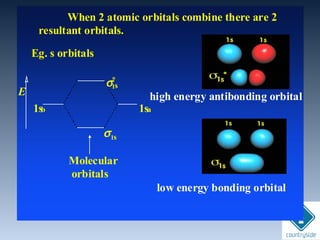
![cA = cB = 1
+. +. . .+
bondingψg
Amplitudes of wave
functions added
ψg = N [ψA + ψB]
Constructive interferenceConstructive interference](https://image.slidesharecdn.com/chemicalbondingandmolecularstructure-130720150057-phpapp02/85/Chemical-bonding-and-molecular-structure-121-320.jpg)
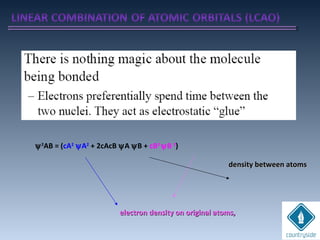
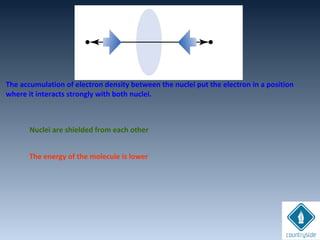
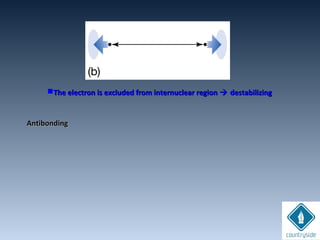
![Amplitudes of wave
functions
subtracted.
Destructive interferenceDestructive interference
Nodal plane perpendicular to the H-H bondNodal plane perpendicular to the H-H bond
axis (en density = 0)axis (en density = 0)
Energy of the en in this orbital is higher.Energy of the en in this orbital is higher.
+. -. ..
node
antibonding
ψu = N [ψA - ψB]
cA = +1, cB = -1 ψu
+ -
ΨA-ΨB](https://image.slidesharecdn.com/chemicalbondingandmolecularstructure-130720150057-phpapp02/85/Chemical-bonding-and-molecular-structure-124-320.jpg)