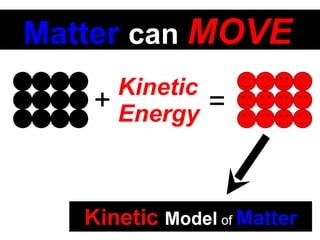
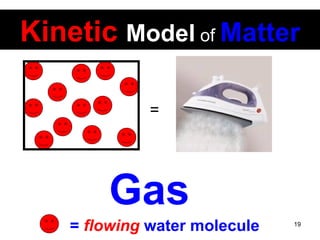
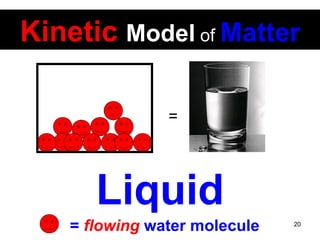
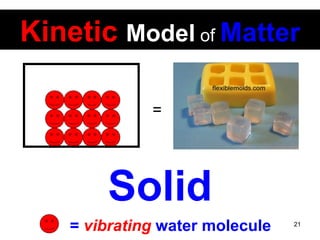
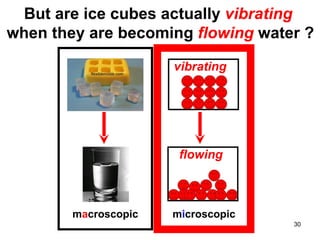
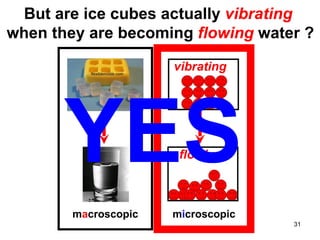
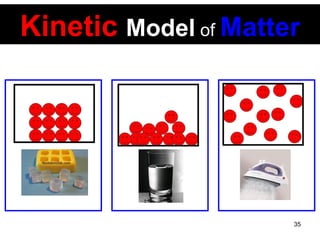
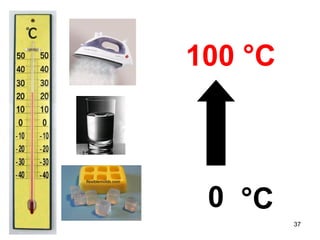
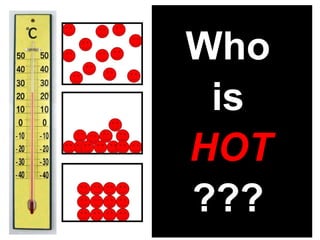
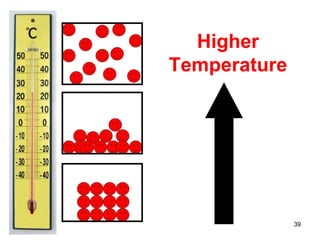
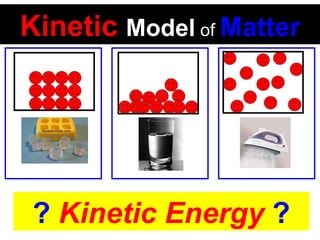
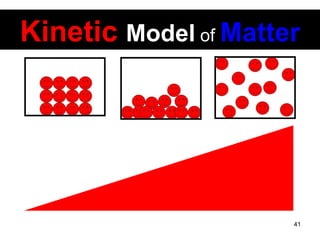
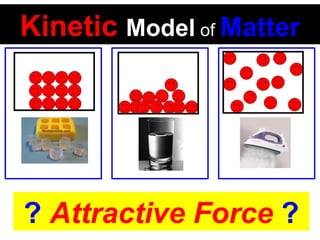
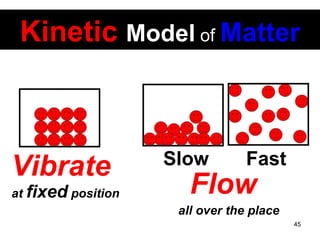
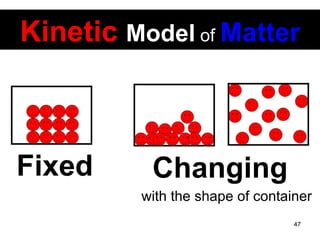
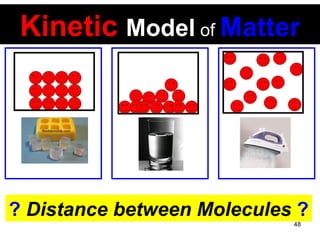
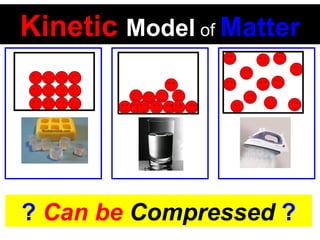
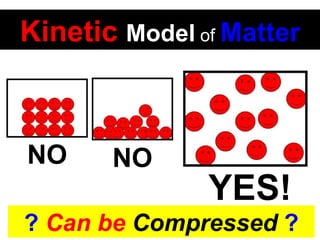
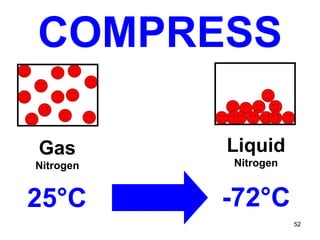
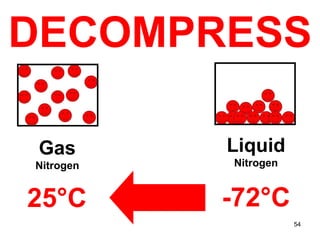
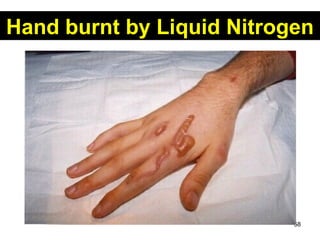
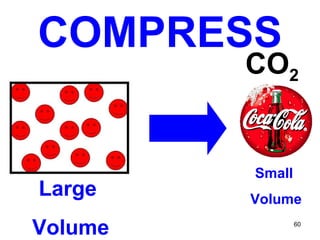
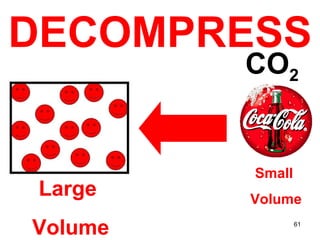
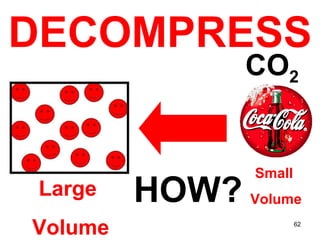
This document discusses the kinetic molecular theory of matter. It explains that all matter is made up of molecules in constant motion. The three states of matter - solid, liquid, and gas - can be described and differentiated based on the motion and arrangement of their molecules. Higher temperatures correspond to higher molecular kinetic energy and faster, more energetic motion. The kinetic theory helps explain various macroscopic properties like compressibility based on molecular-level behavior and interactions.