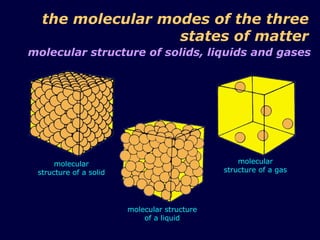
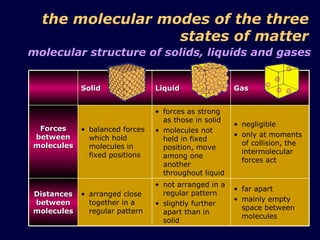
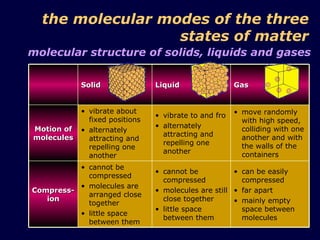
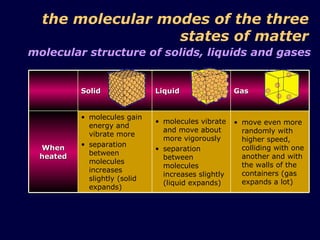
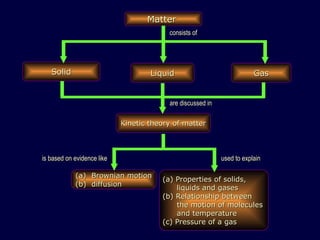
1. The kinetic theory of matter states that all matter is made up of tiny particles called atoms and molecules that are in continuous random motion.
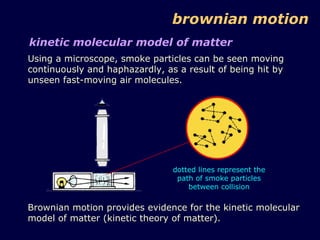
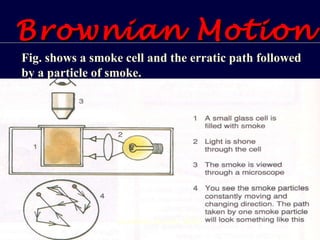
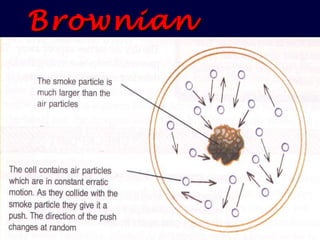
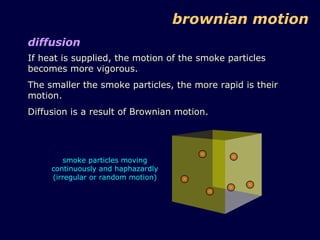
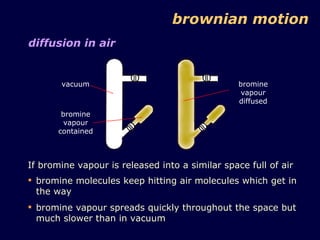
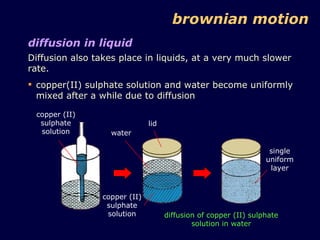
2. Brownian motion provides evidence for this theory by showing the random movement of small particles suspended in a fluid under a microscope.
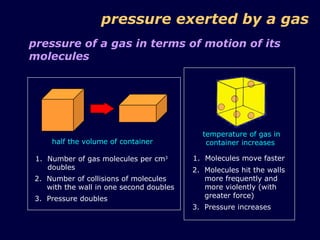
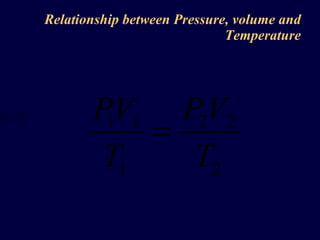
3. The pressure exerted by a gas is caused by collisions of the gas molecules with the walls of their container, and increases when the temperature rises or volume decreases due to the more vigorous motion of the molecules.