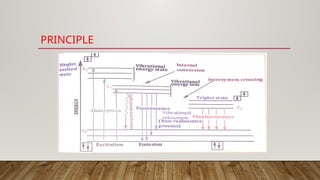
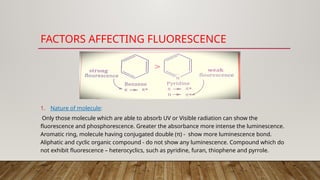
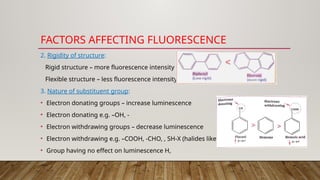
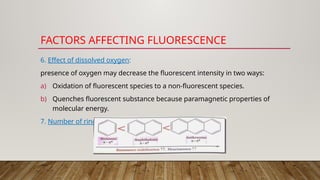
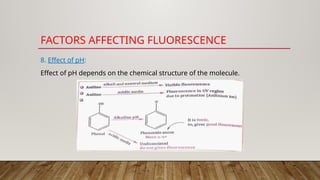
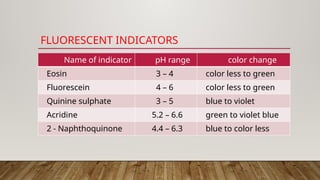
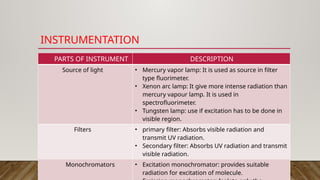
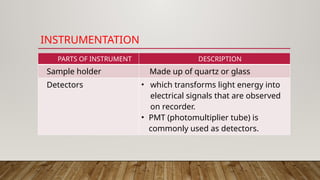
Fluorimetry, or fluorescence spectrometry, is an analytical technique that measures the intensity of light emitted by a substance after absorbing specific wavelengths of light, leading to phenomena such as fluorescence and phosphorescence. The document discusses the theory, principles, factors affecting fluorescence including molecular structure, temperature, and pH, as well as the instrumentation involved in fluorimetry. Applications include the determination of various inorganic substances and detecting impurities at nanogram levels.