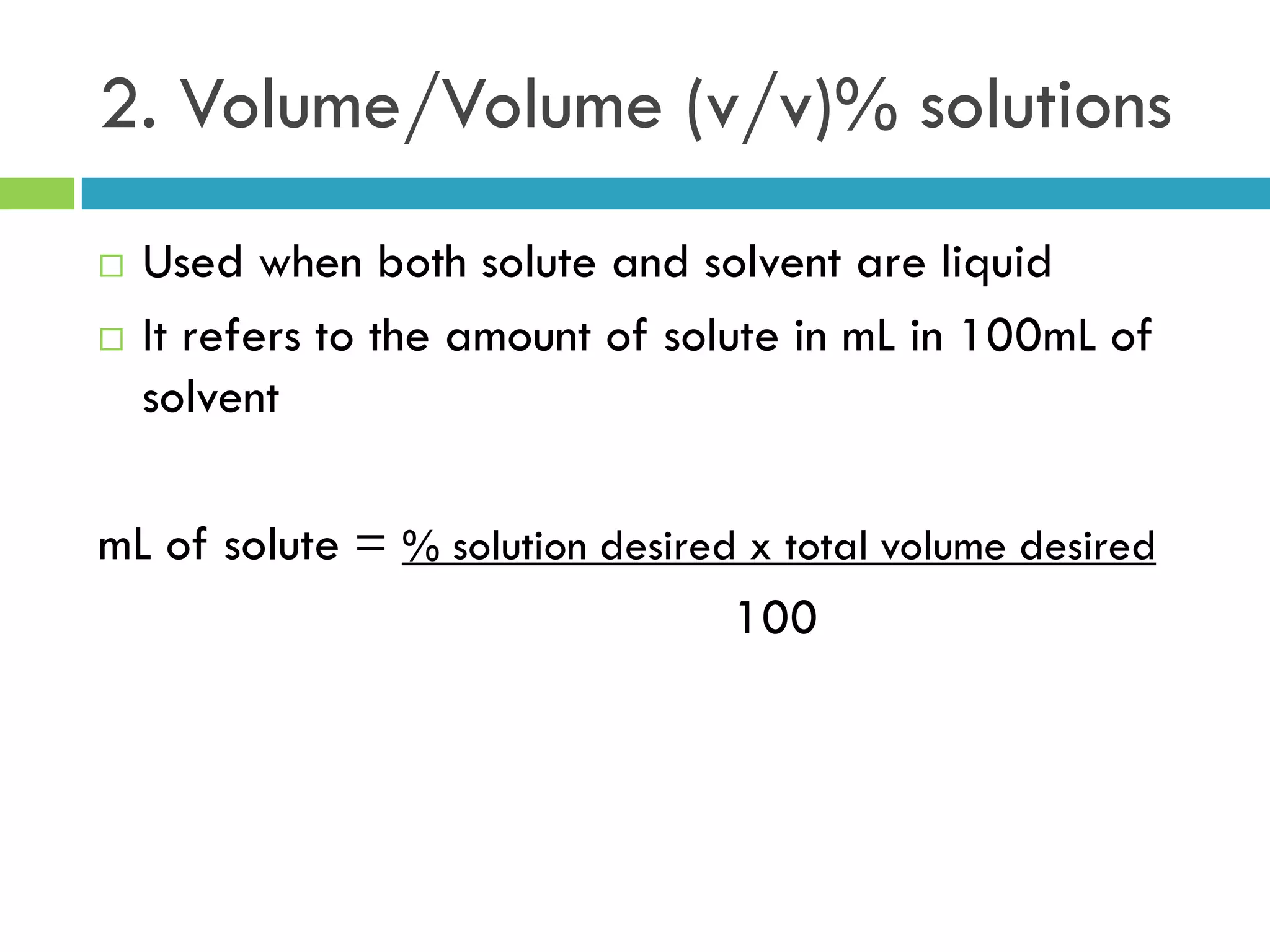
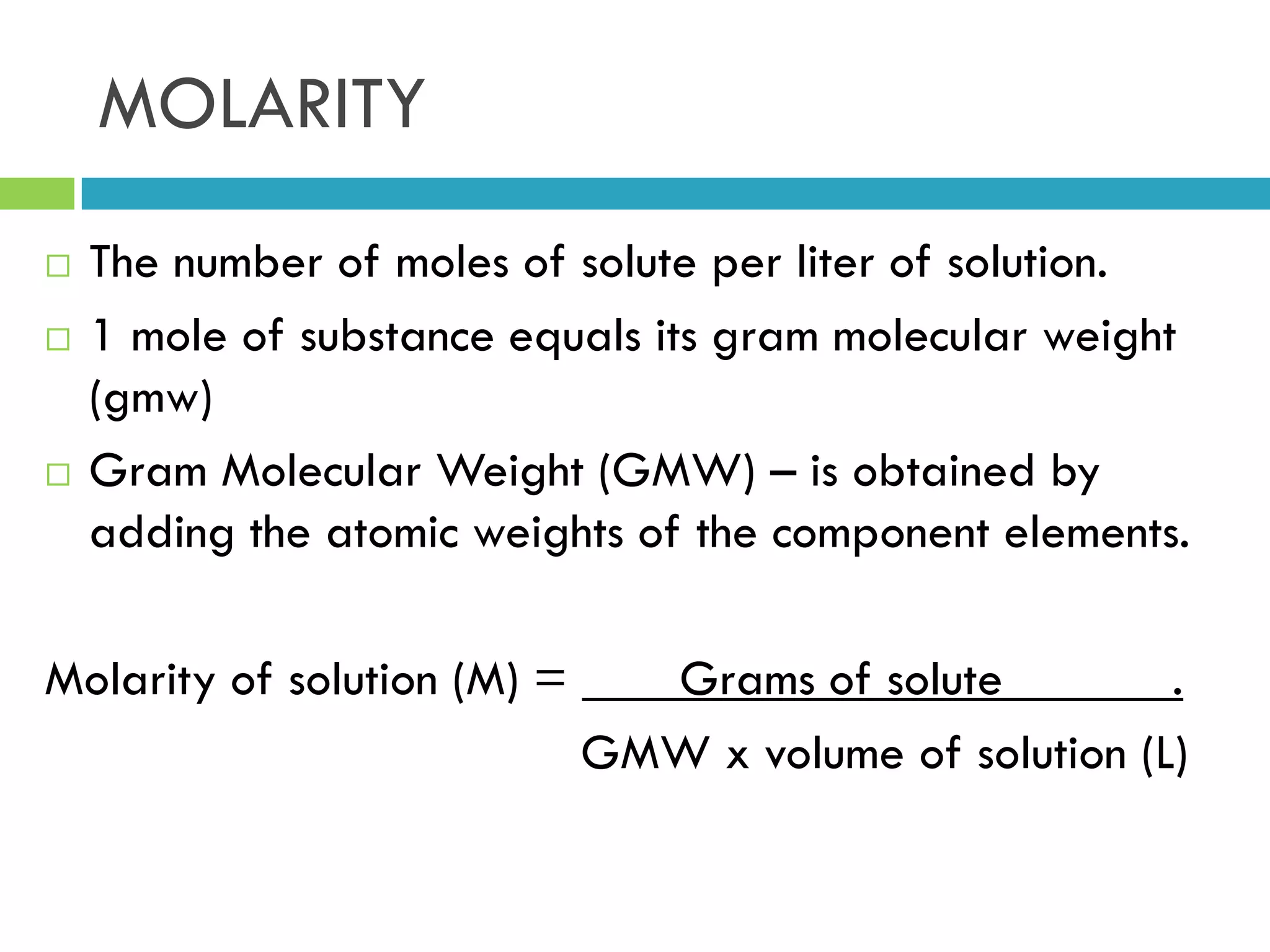
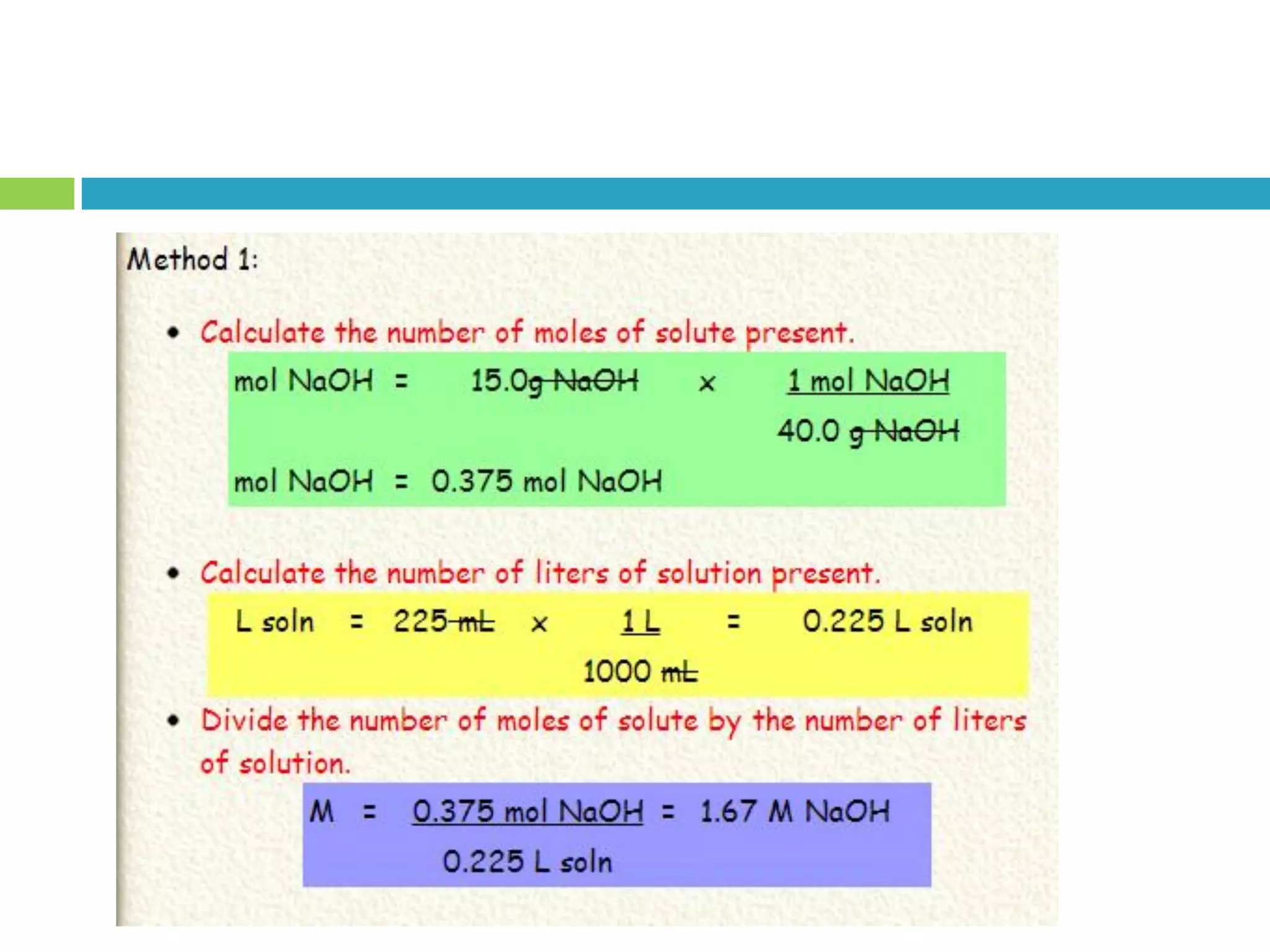
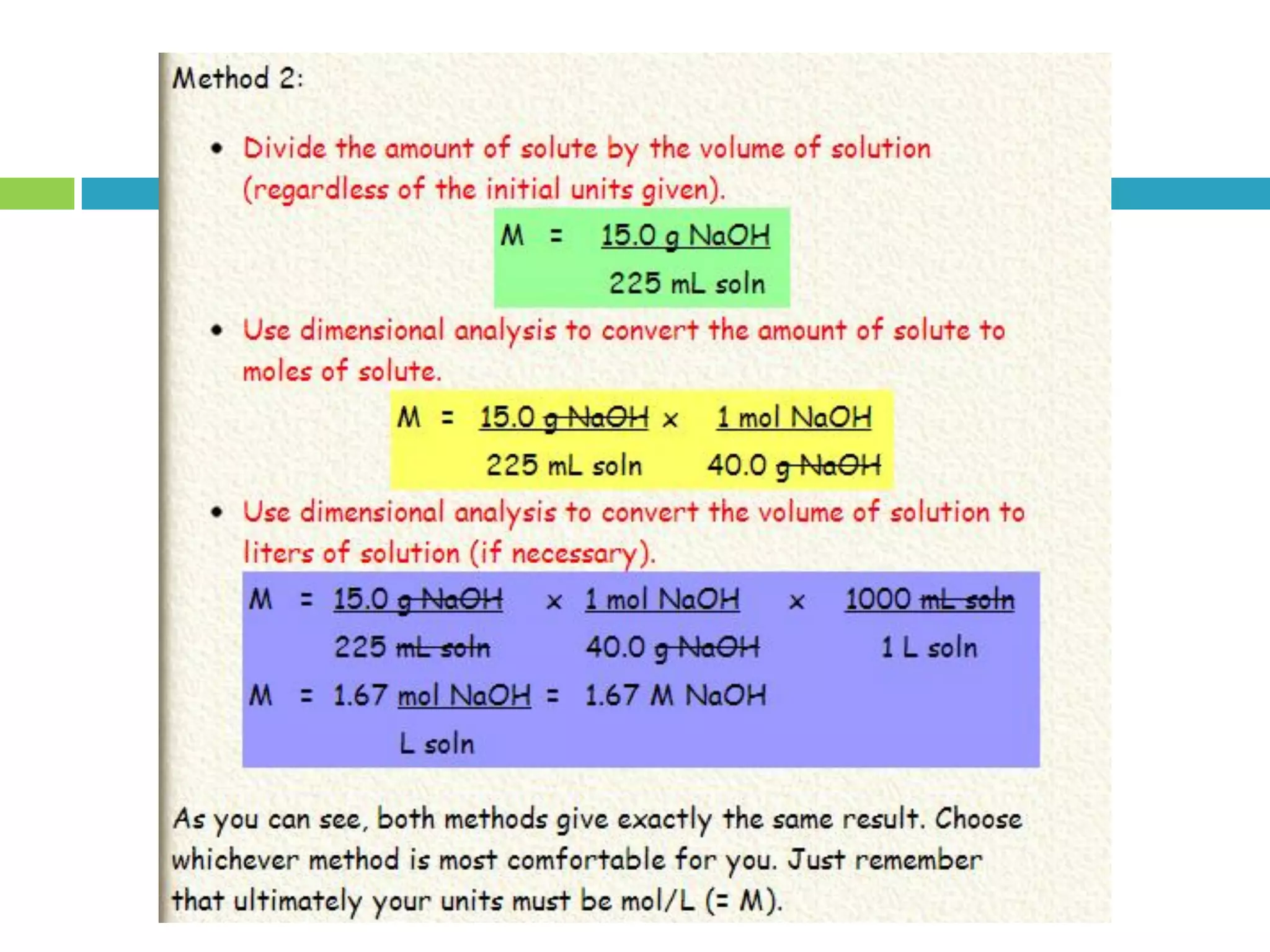
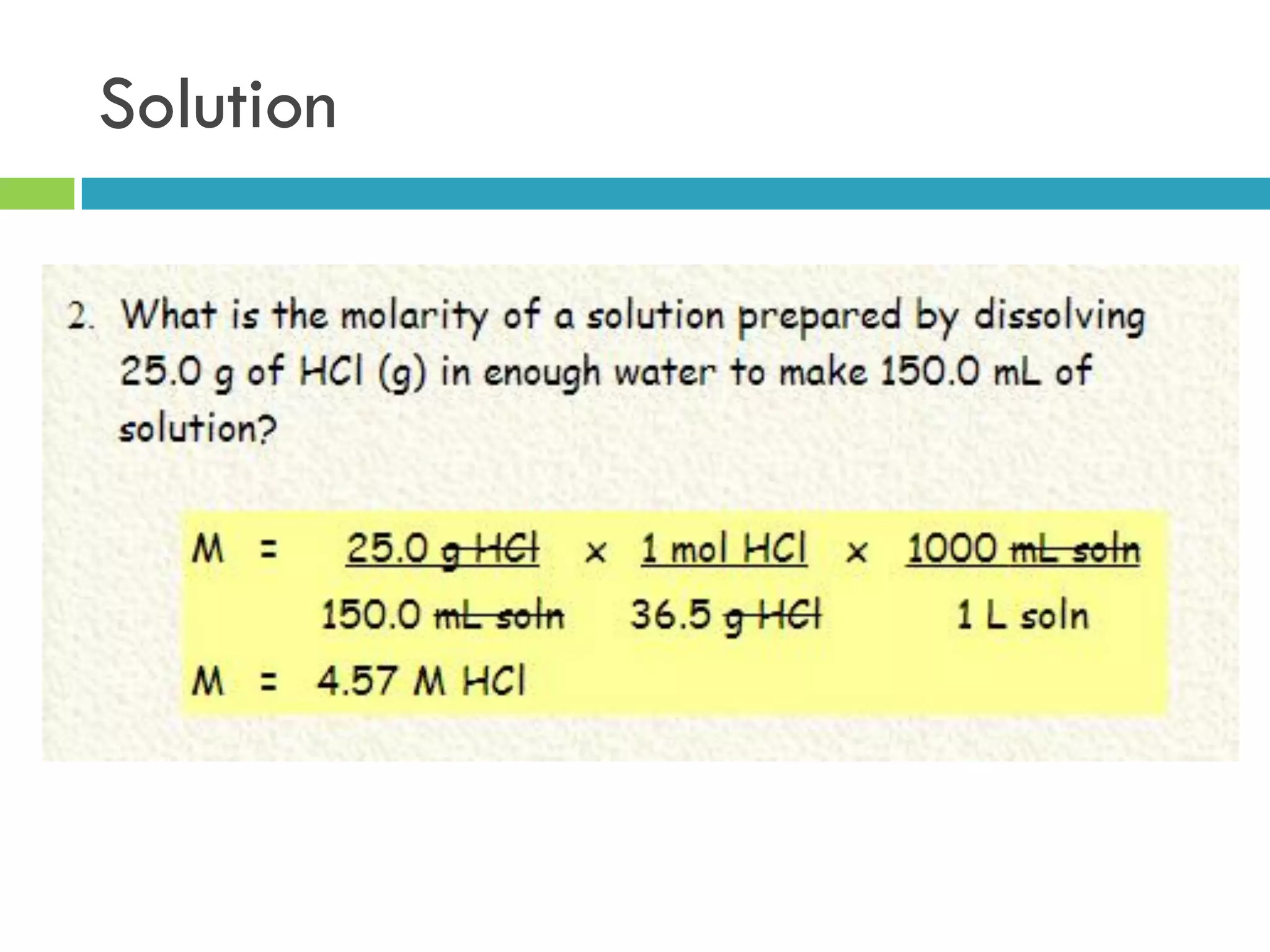
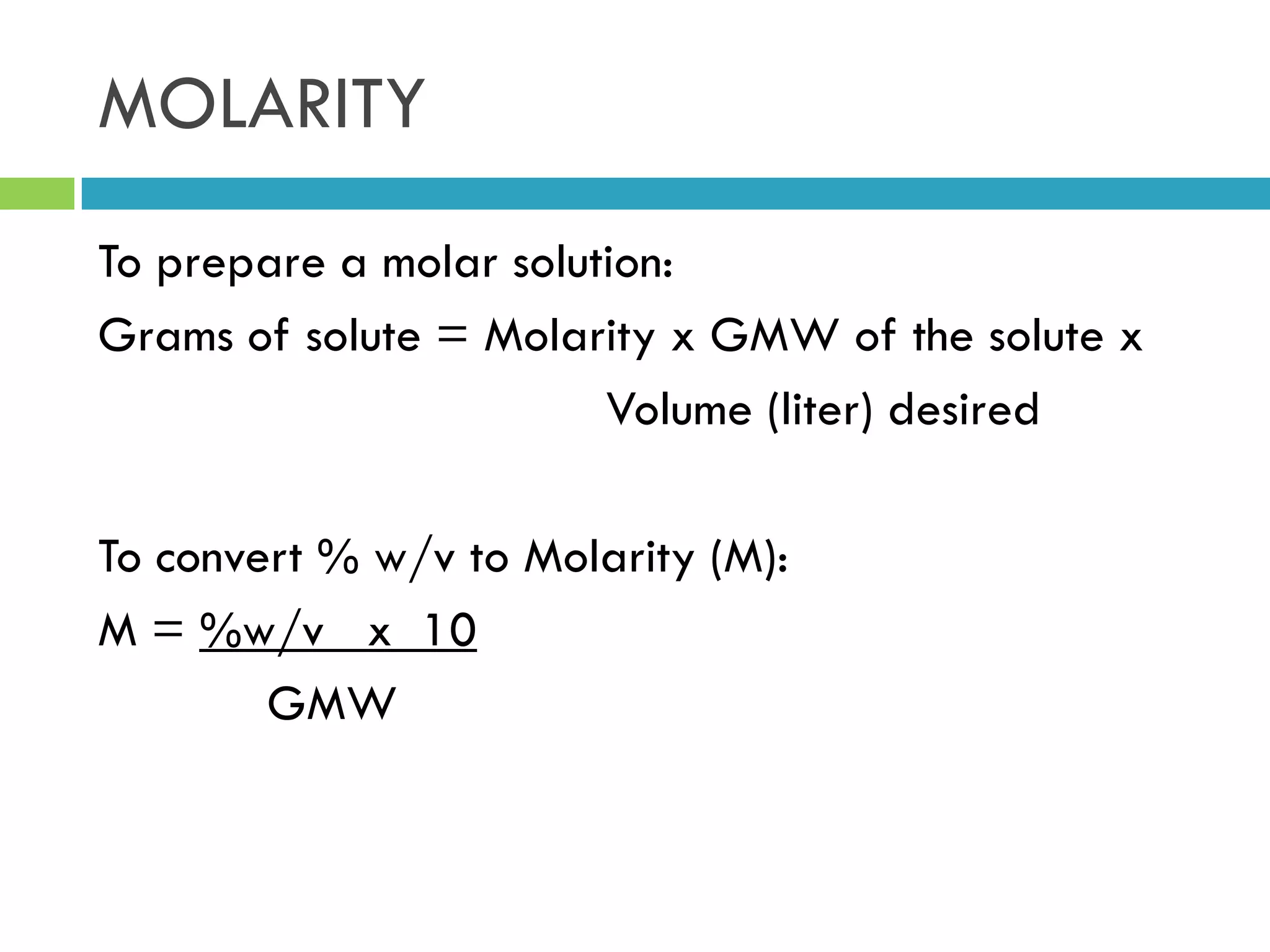
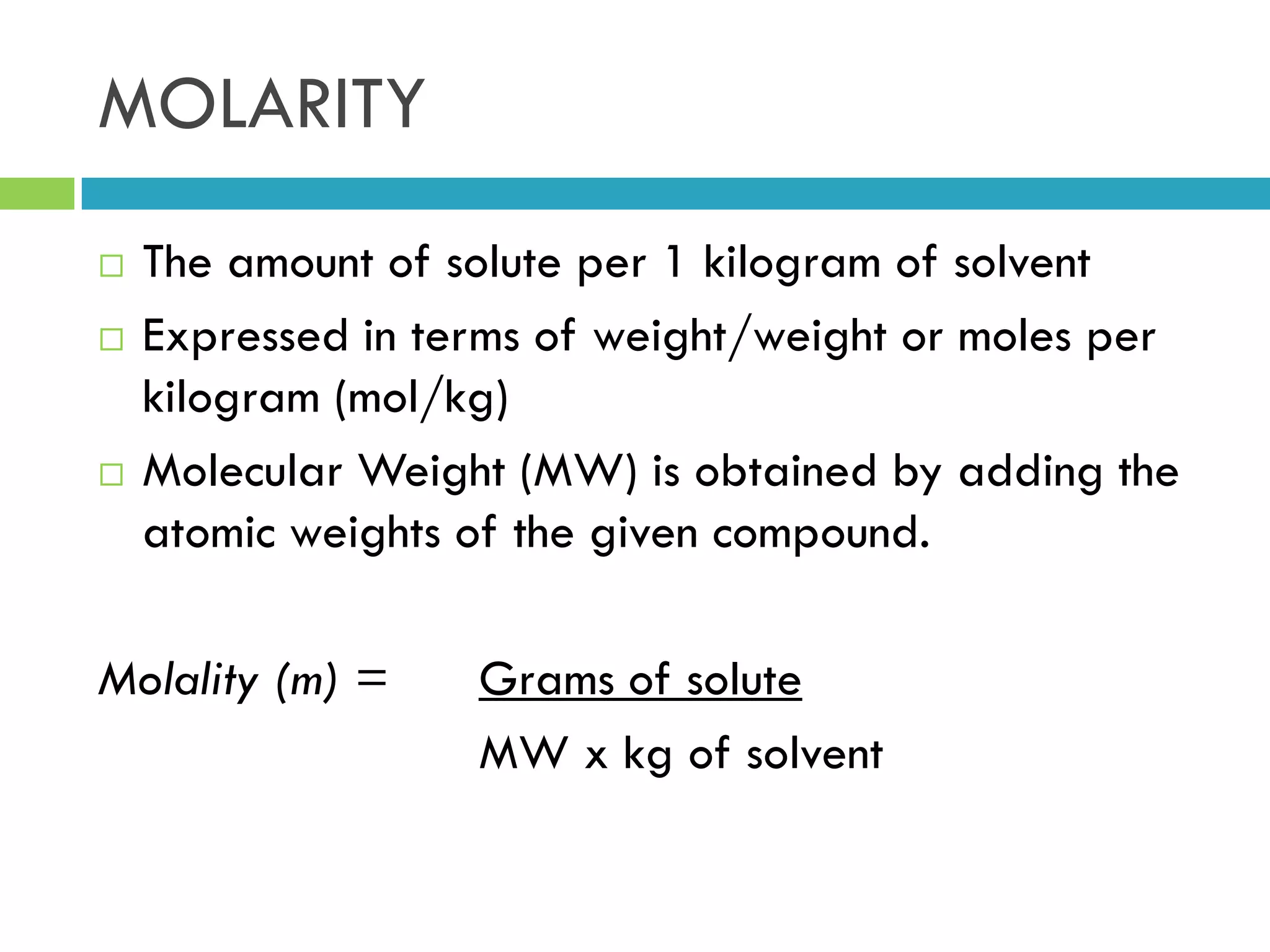
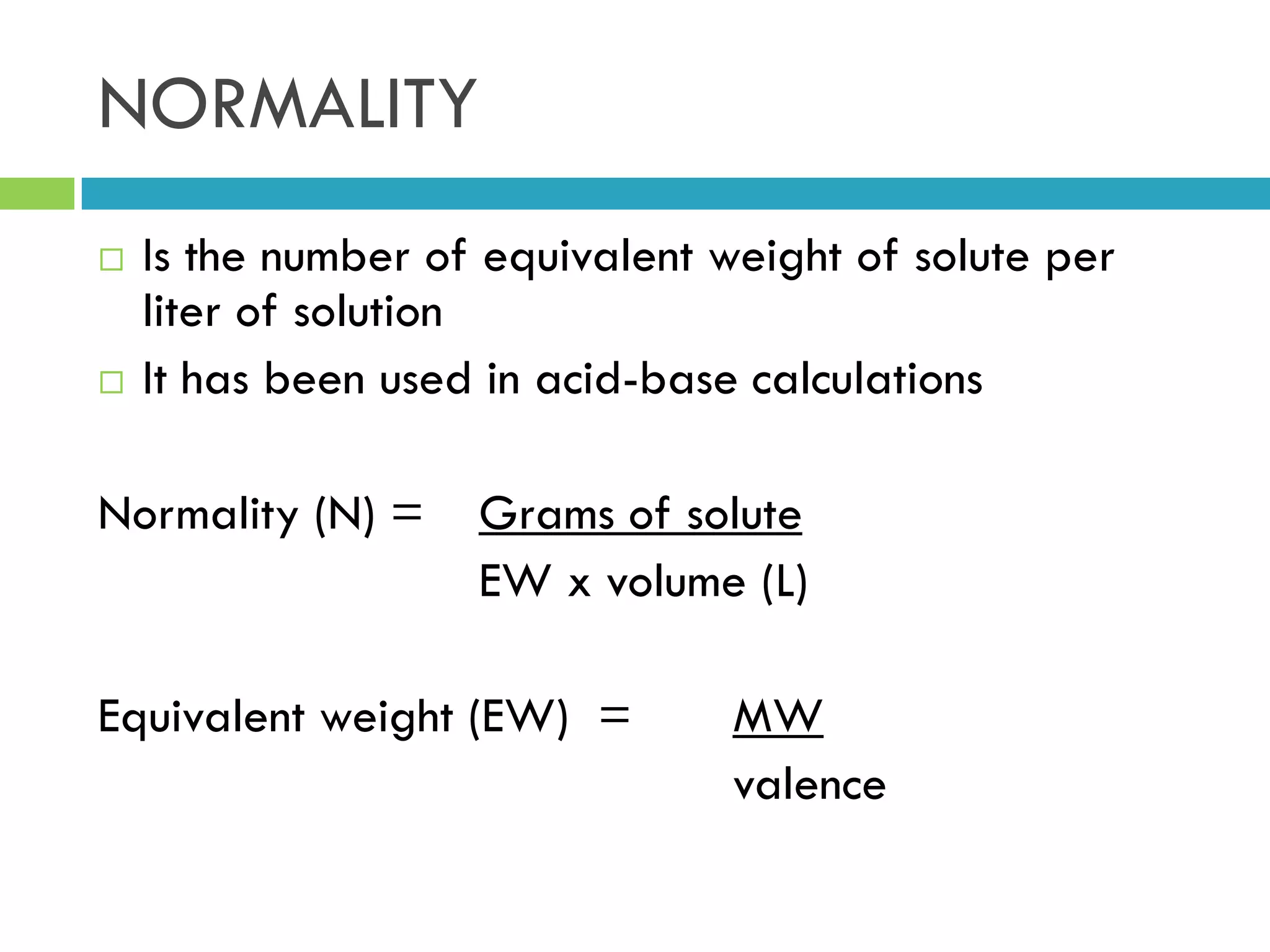
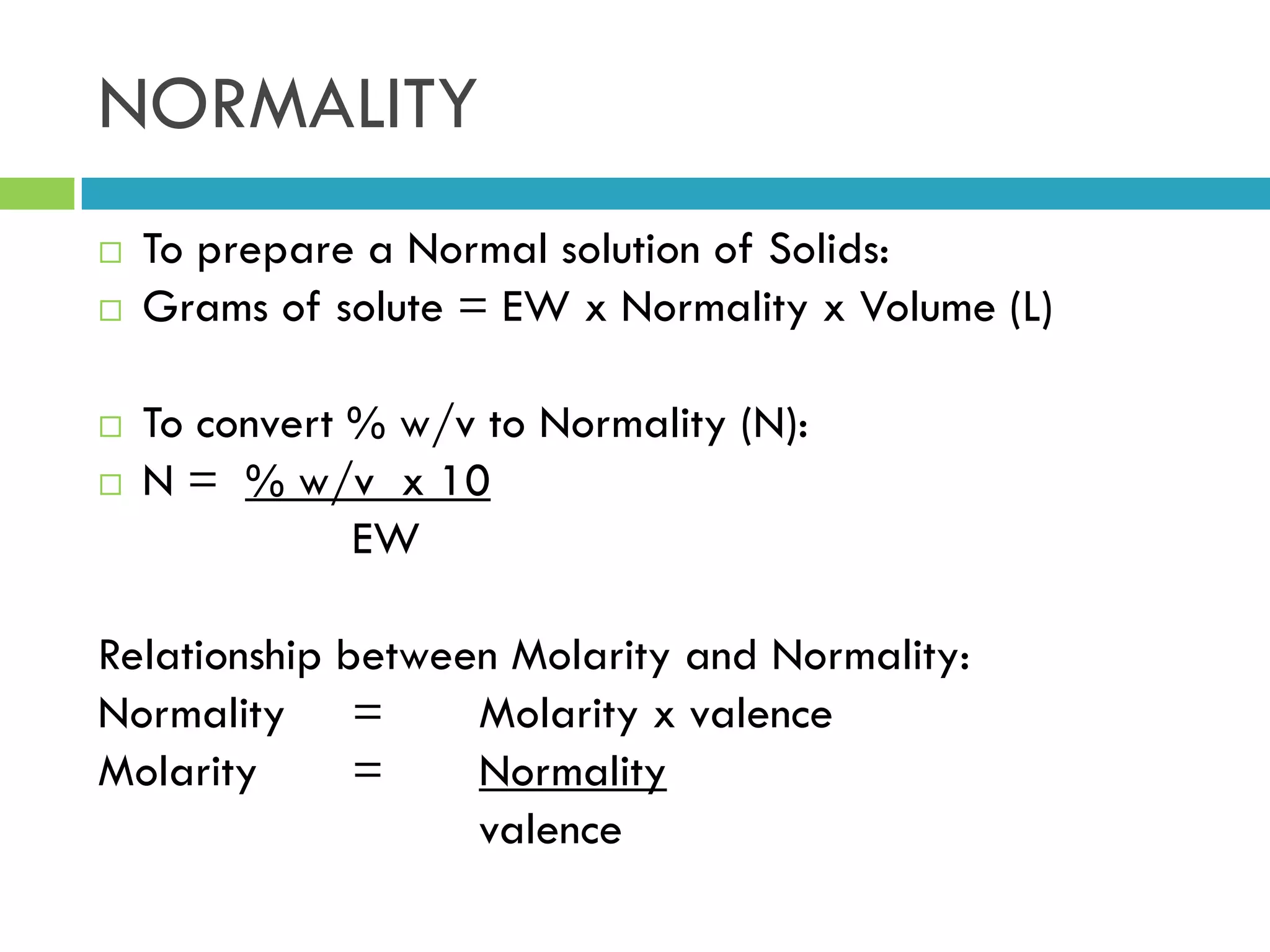
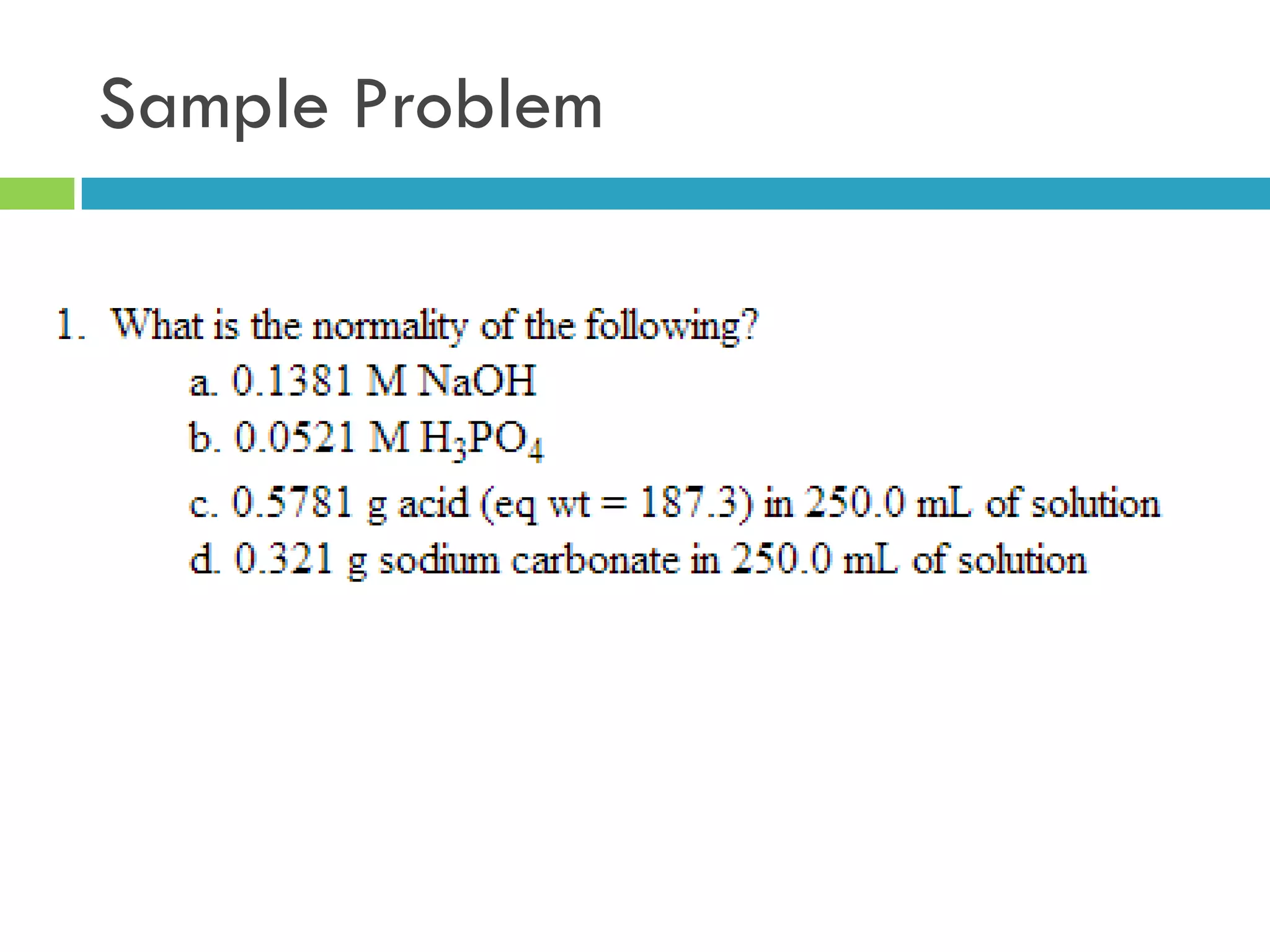
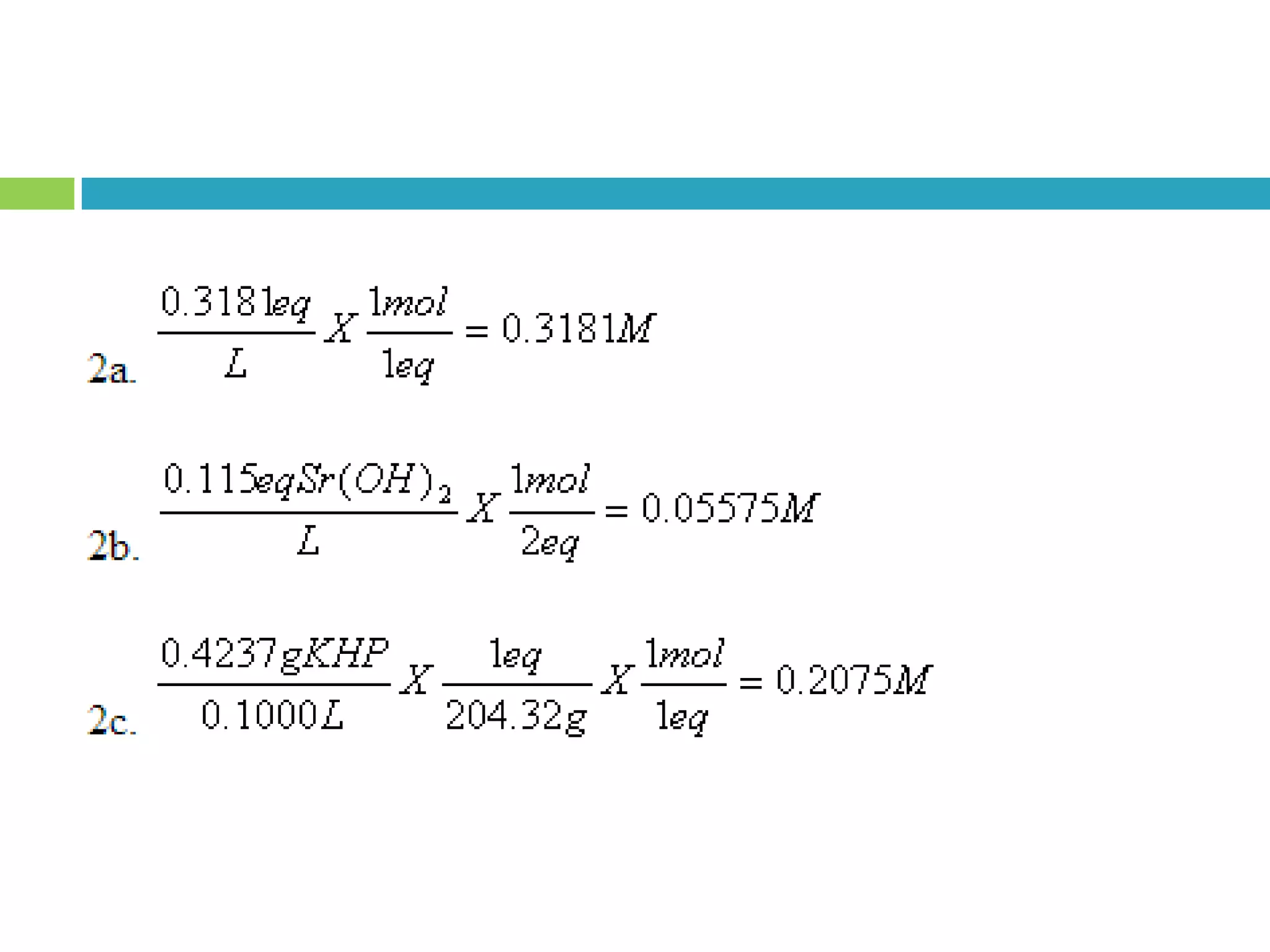
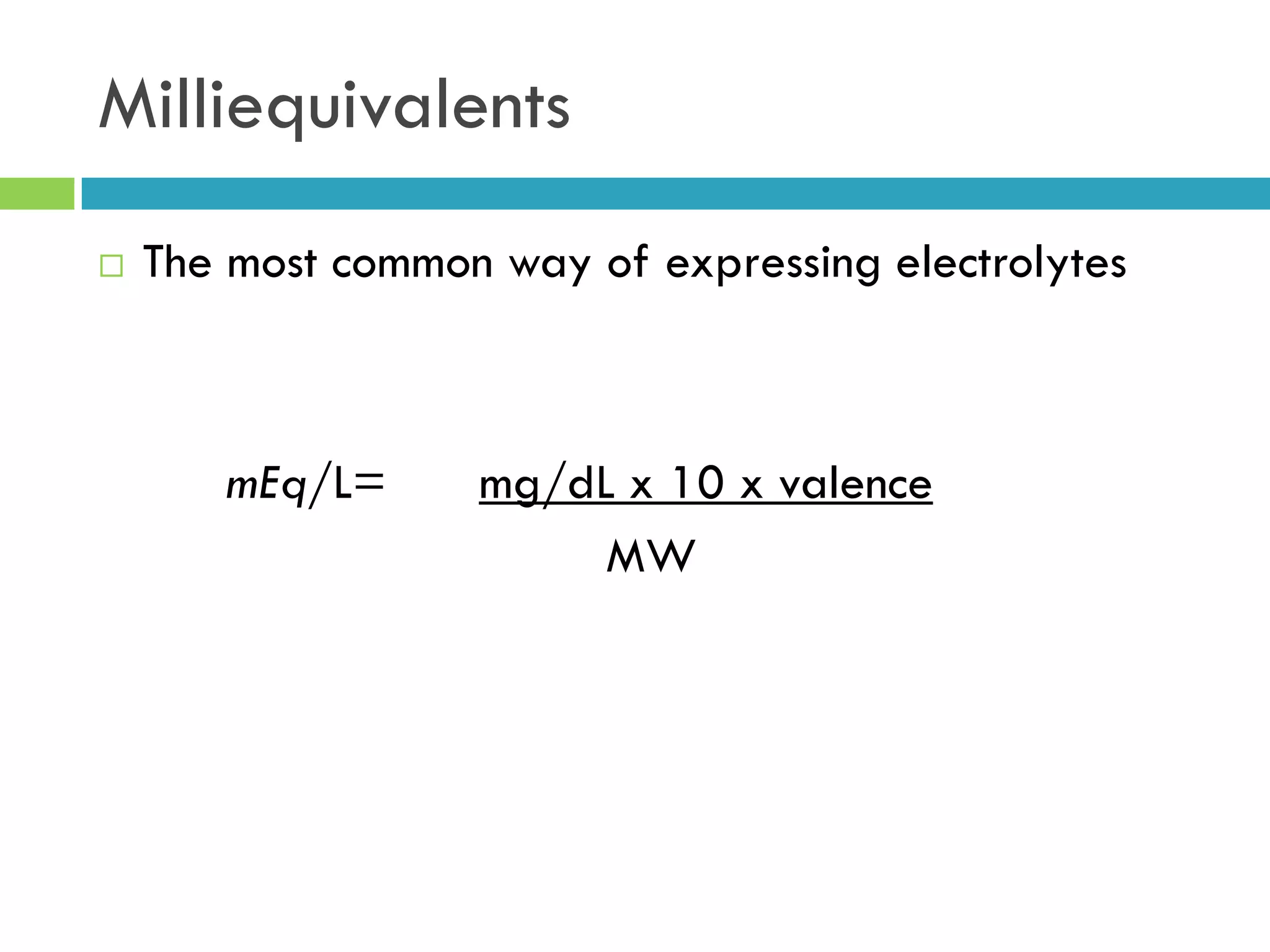
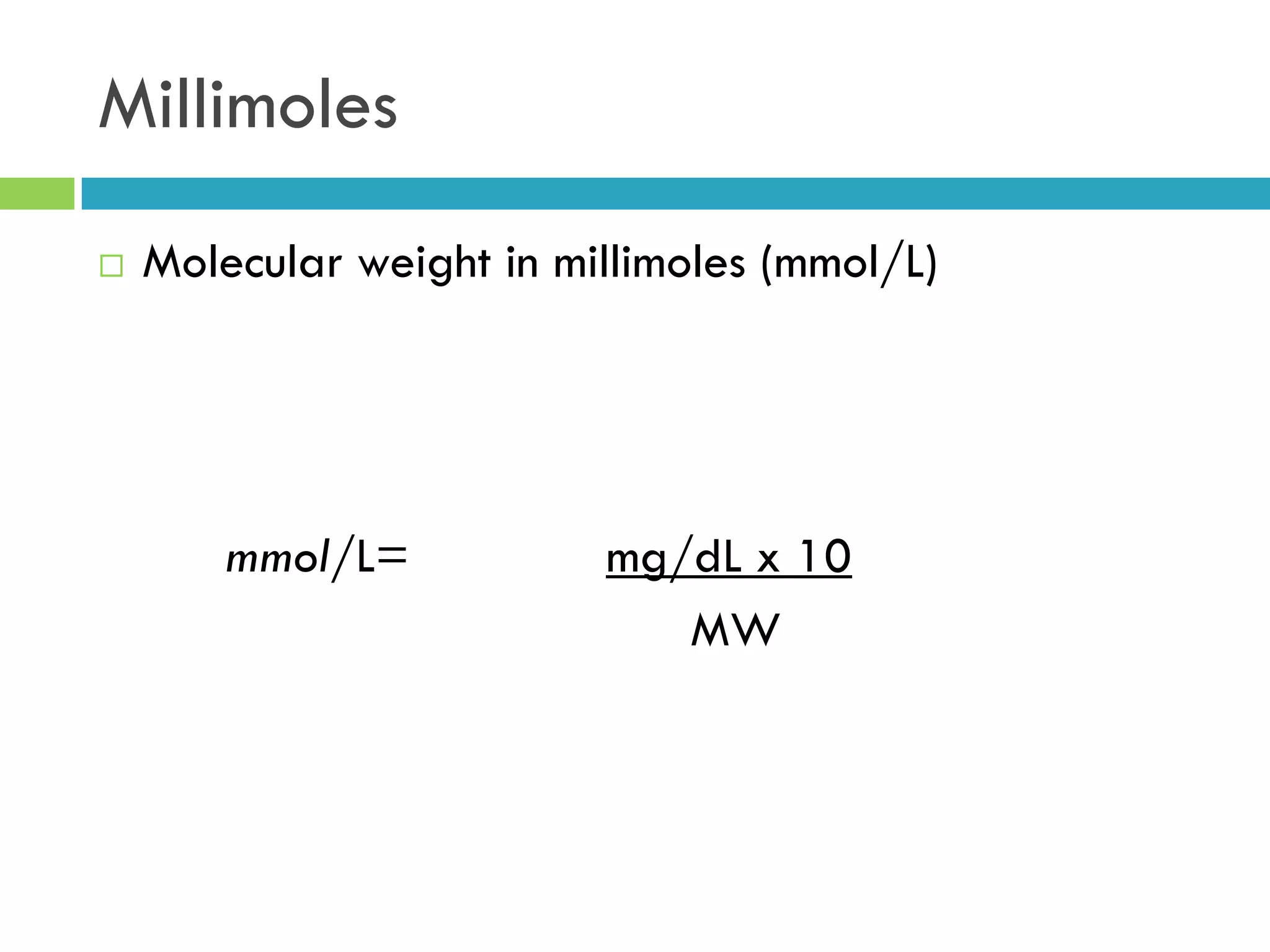
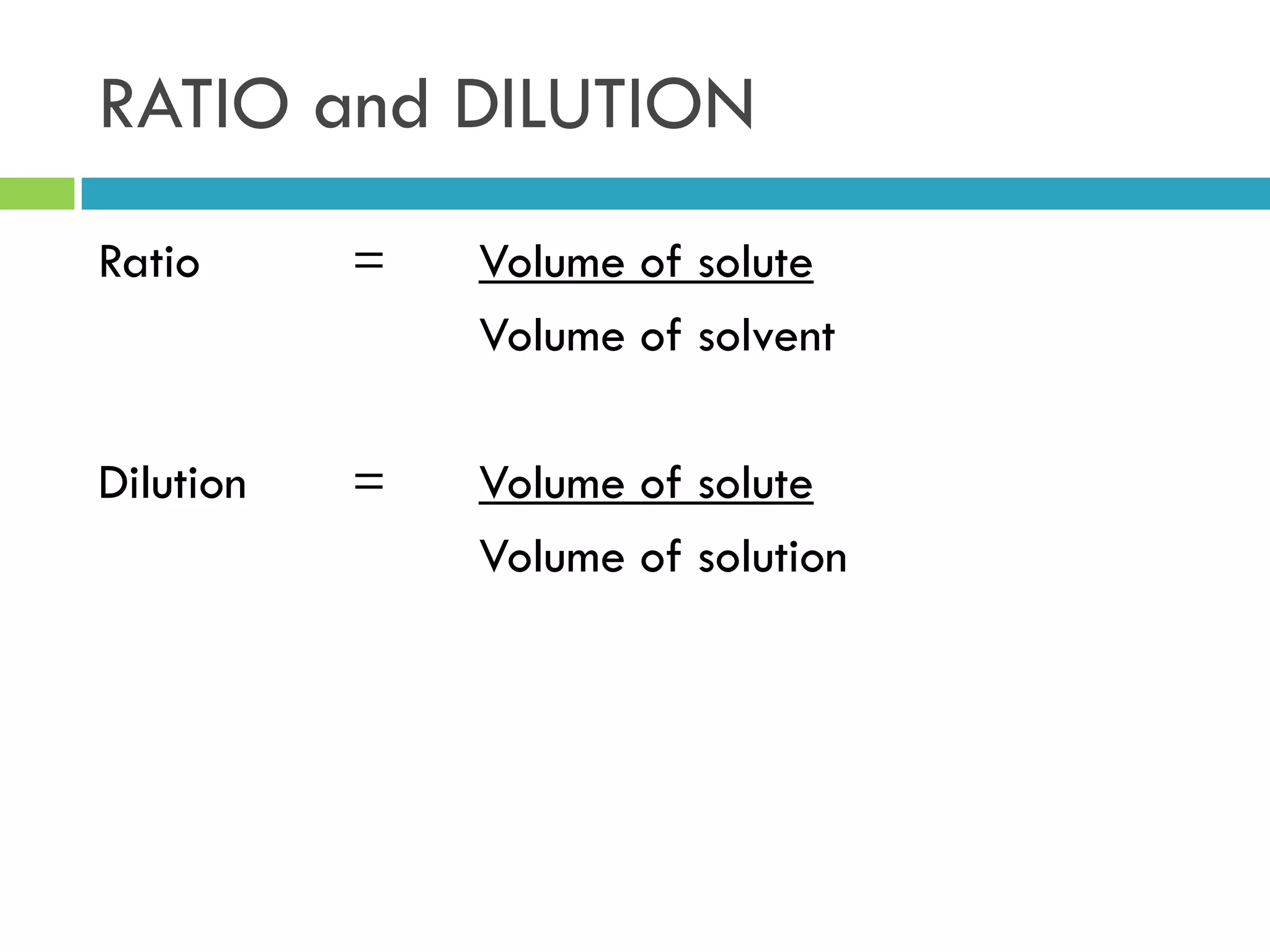
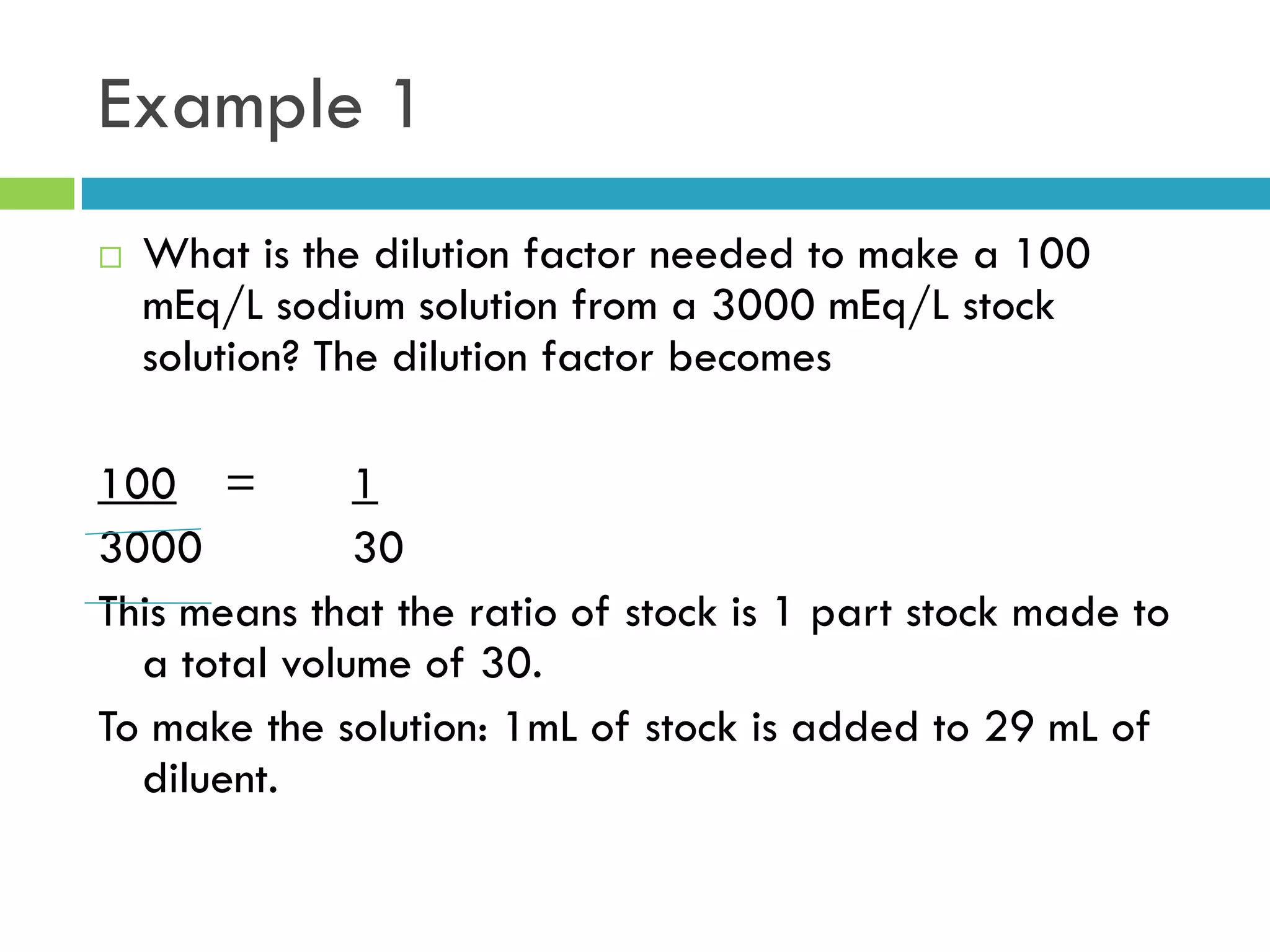
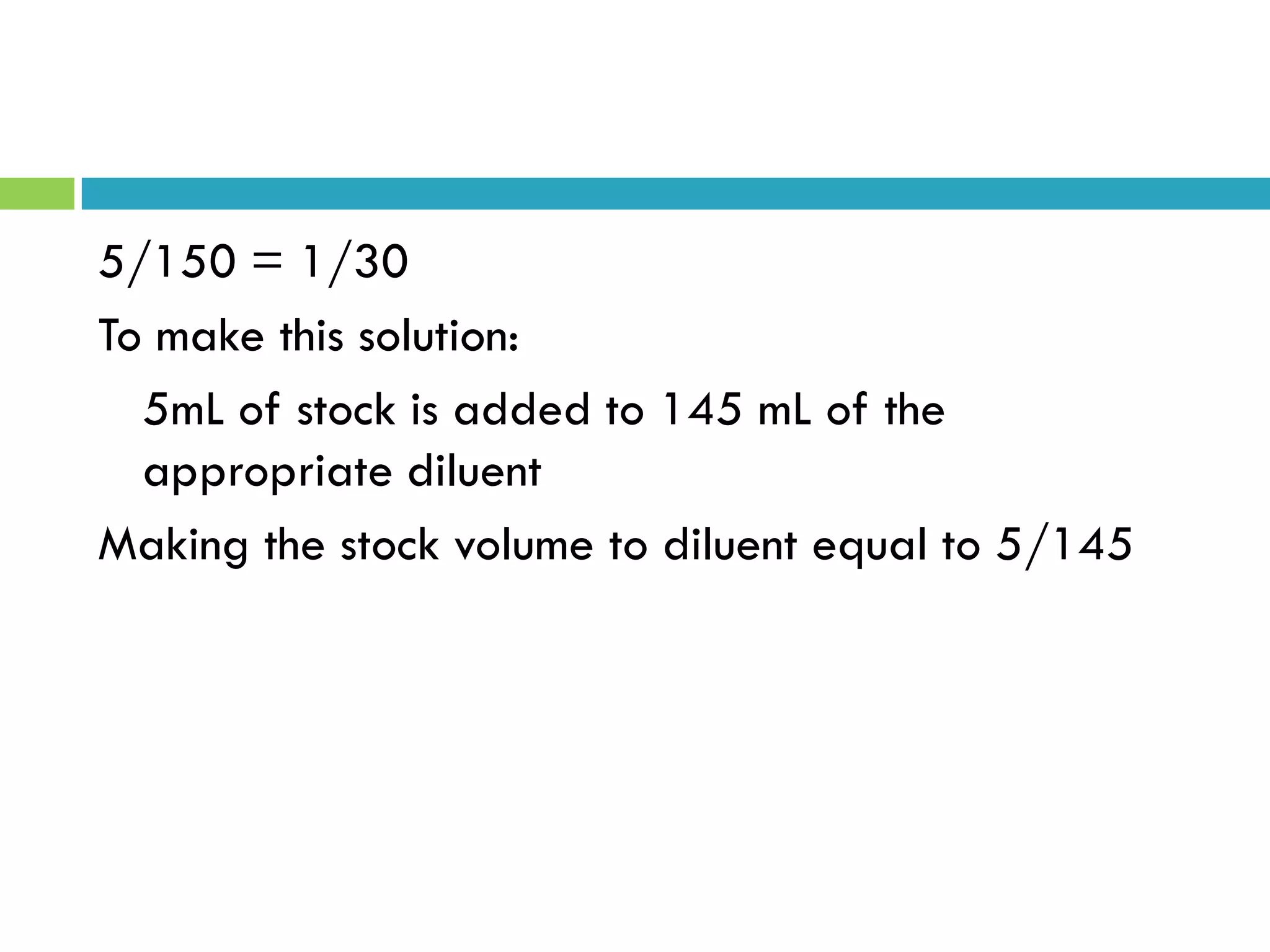
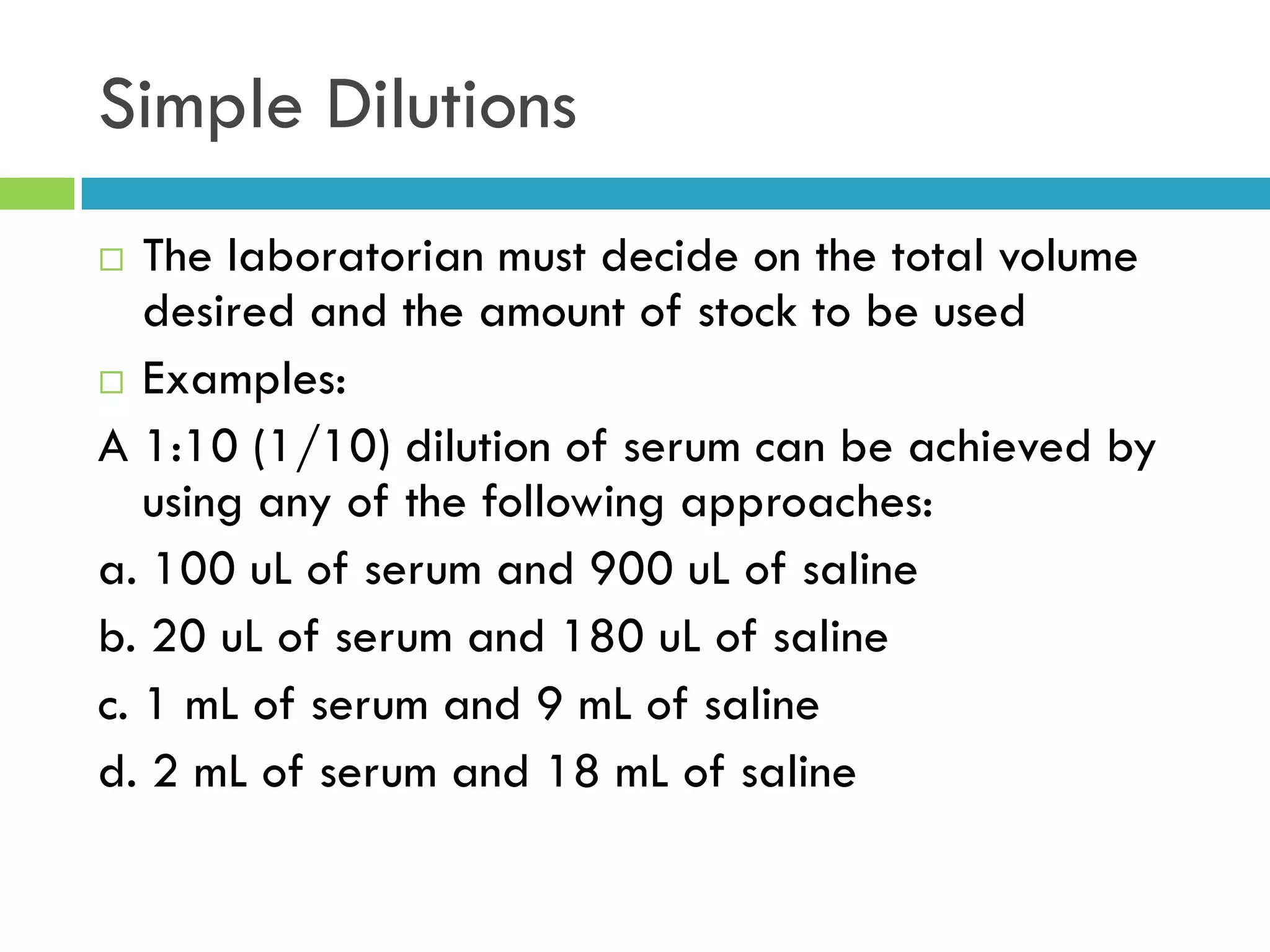
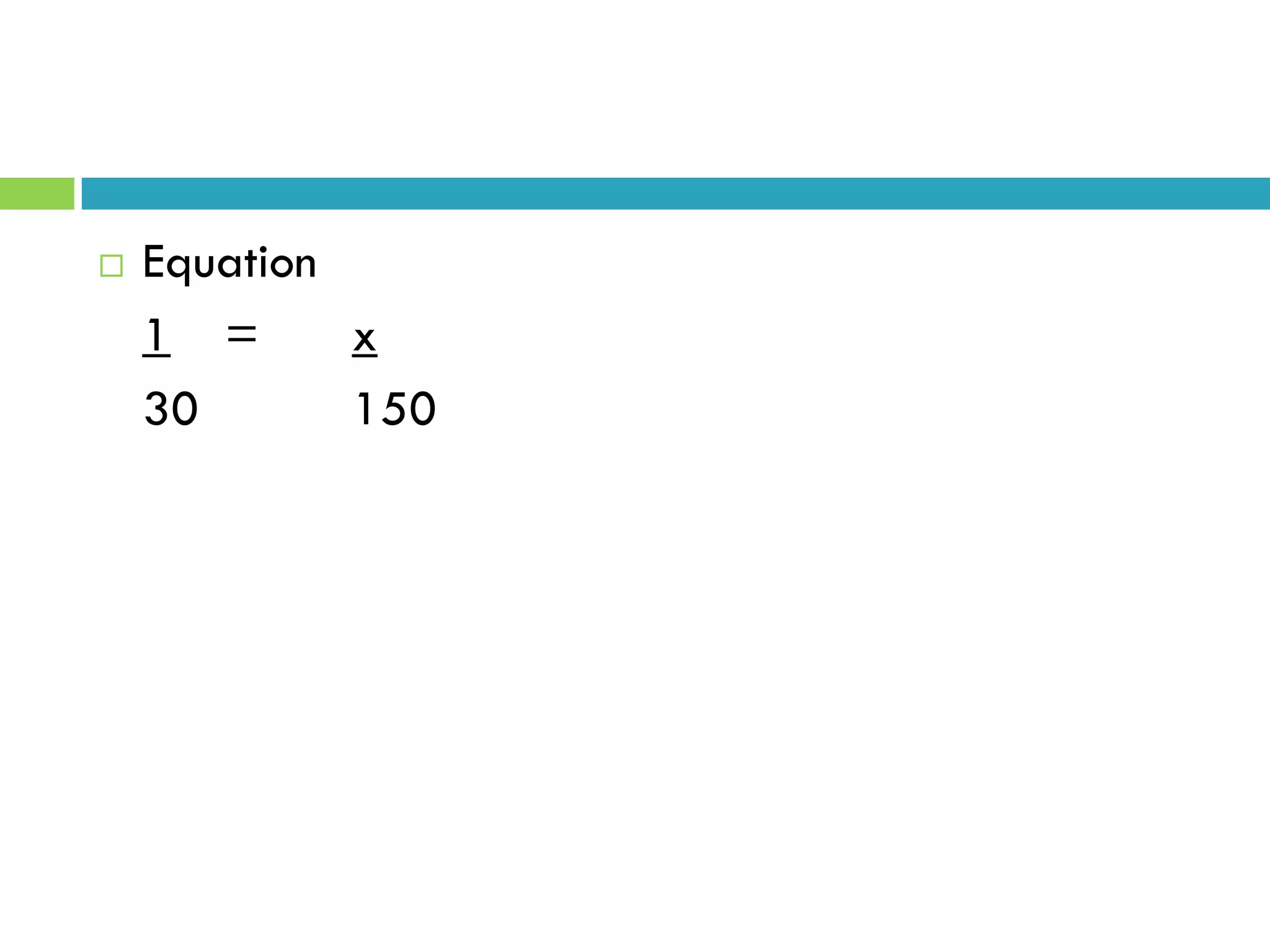This document provides instructions on proper pipetting techniques using manual pipettes and automatic micropipettors. It discusses developing good pipetting skills through practice to avoid accidents. It also covers pipetting steps, reading the meniscus, diluting solutions, and performing calculations related to molarity, normality, milliequivalents, and dilution factors.