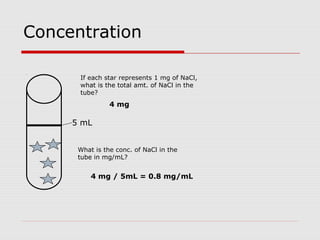
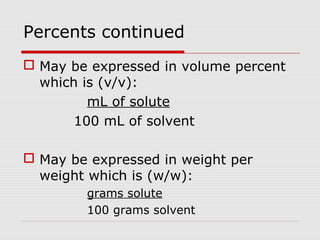
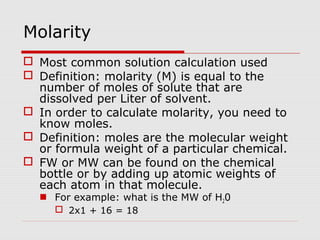
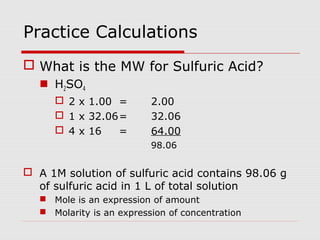
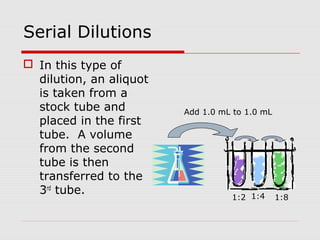
This document discusses important concepts for preparing solutions in biotechnology. It defines key terms like solute, solvent, concentration, and solution. It explains different units for expressing concentration, such as weight/volume percent, molarity, and normality. Examples are provided for calculating amounts of solute needed to make solutions of a given concentration and volume. Equations are presented for diluting stock solutions to prepare solutions of lower concentration. Serial dilutions are also introduced as a method to make a series of progressively more dilute solutions from a concentrated stock.