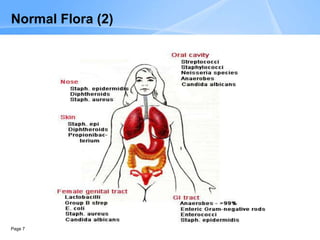
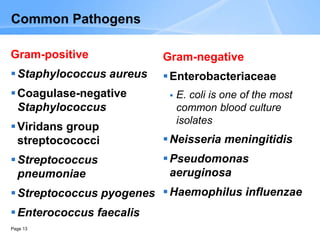
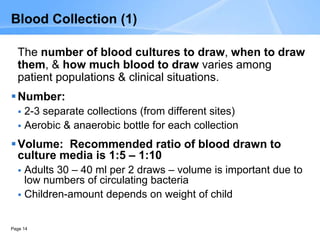
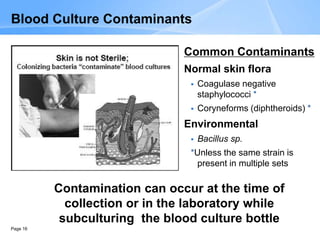
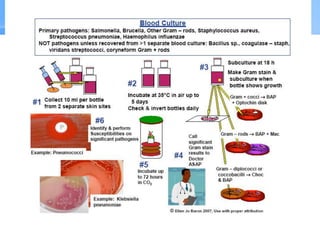
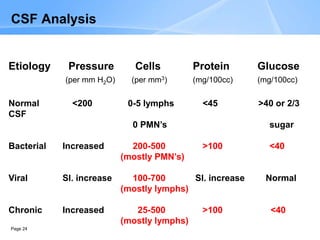
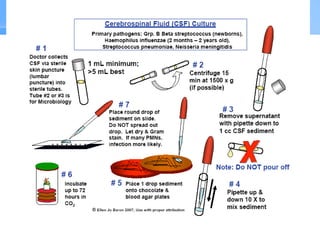
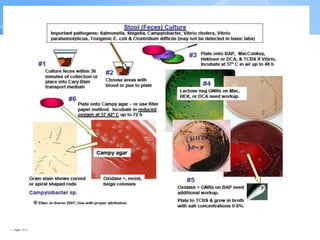
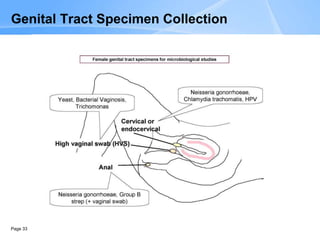
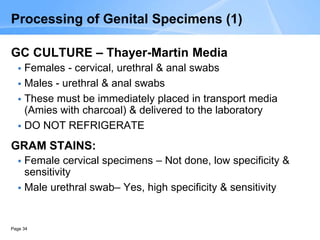
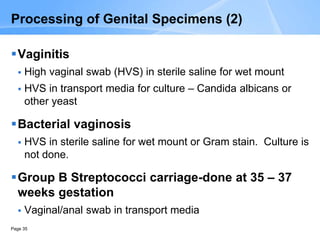
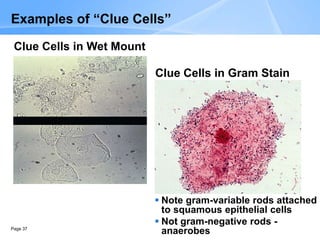
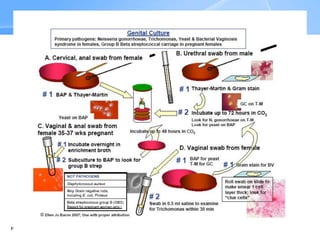
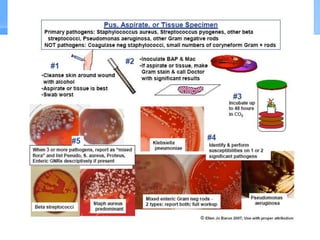
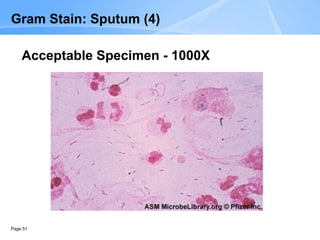
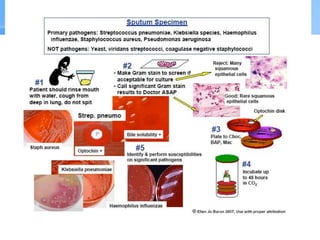
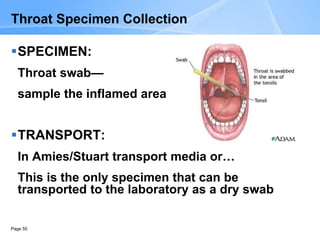
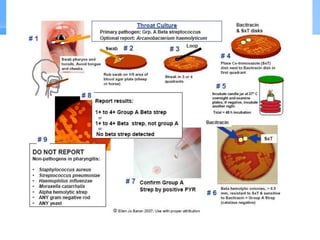
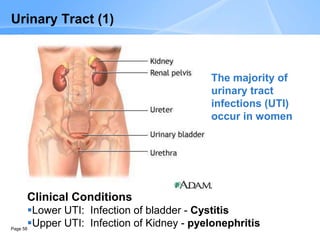
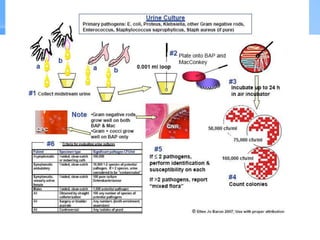
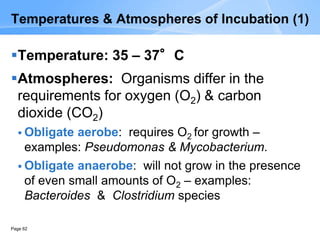
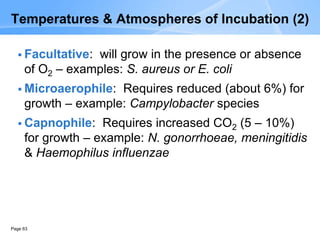
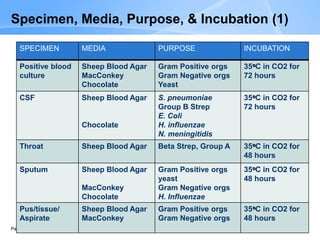
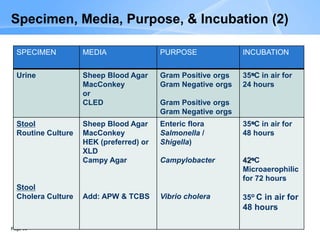
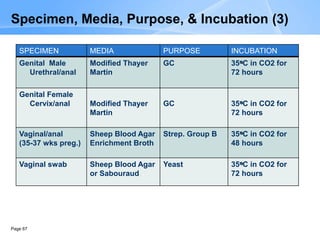
This document provides an overview of specimen processing in a clinical microbiology laboratory. It covers the normal flora, opportunistic infections, and nosocomial infections that can be found in different specimen types, including blood, cerebrospinal fluid, stool, genital, wound/tissue, sputum, throat, and urine samples. For each specimen type, it describes the relevant clinical conditions, common pathogens, collection and transport methods, and processing including any special culture techniques or stains used.