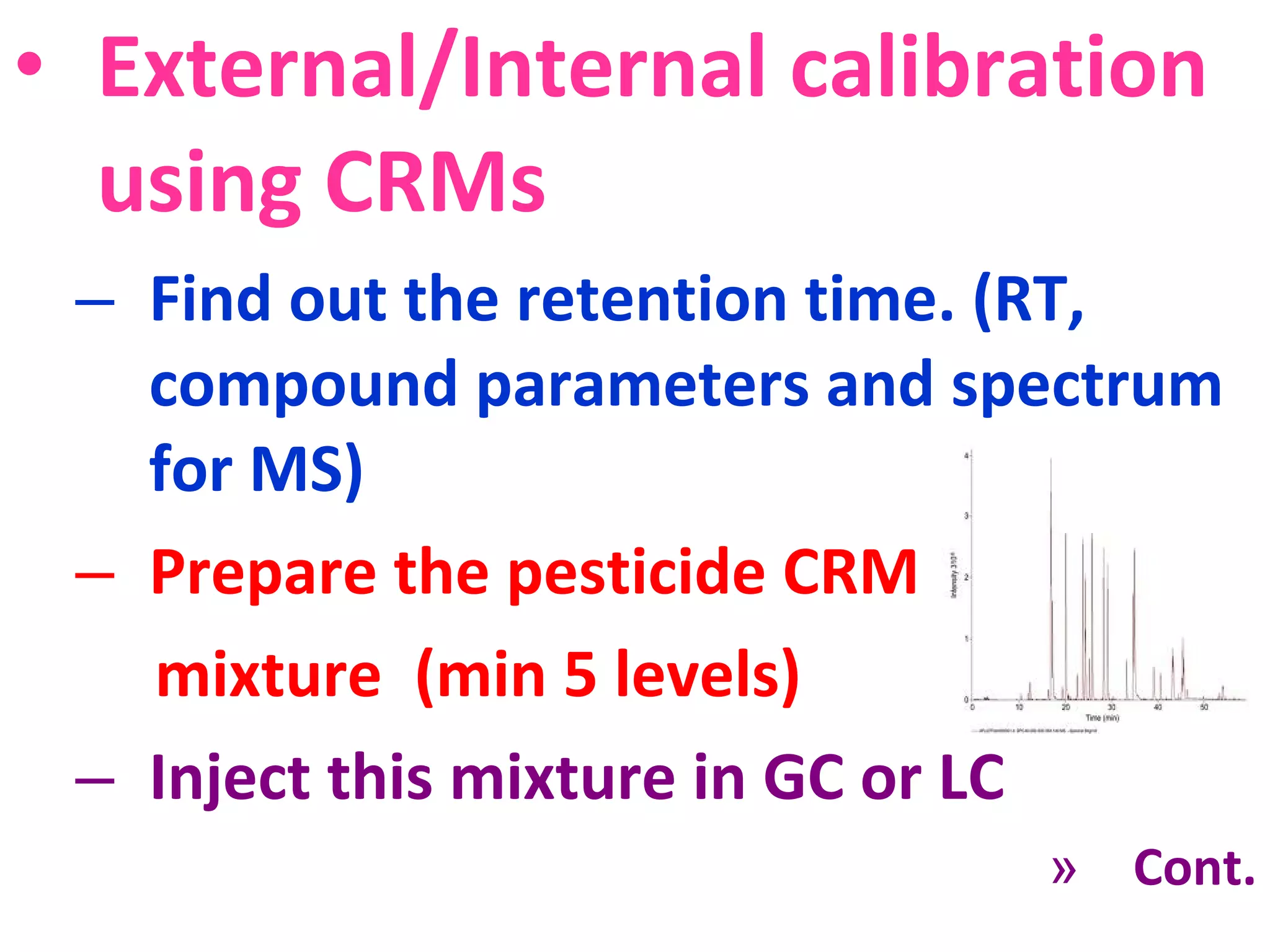
Calibration establishes the relationship between instrument measurements and known standard values through a series of steps. Key aspects of calibration include identifying instruments and sources, following calibration procedures, documenting results, accounting for sources of error, and ensuring traceability to national standards. Calibration procedures vary based on instrument type, but generally involve evaluating instrument performance, establishing calibration curves using certified reference materials at multiple concentration levels, and quantifying samples based on the calibration curves.