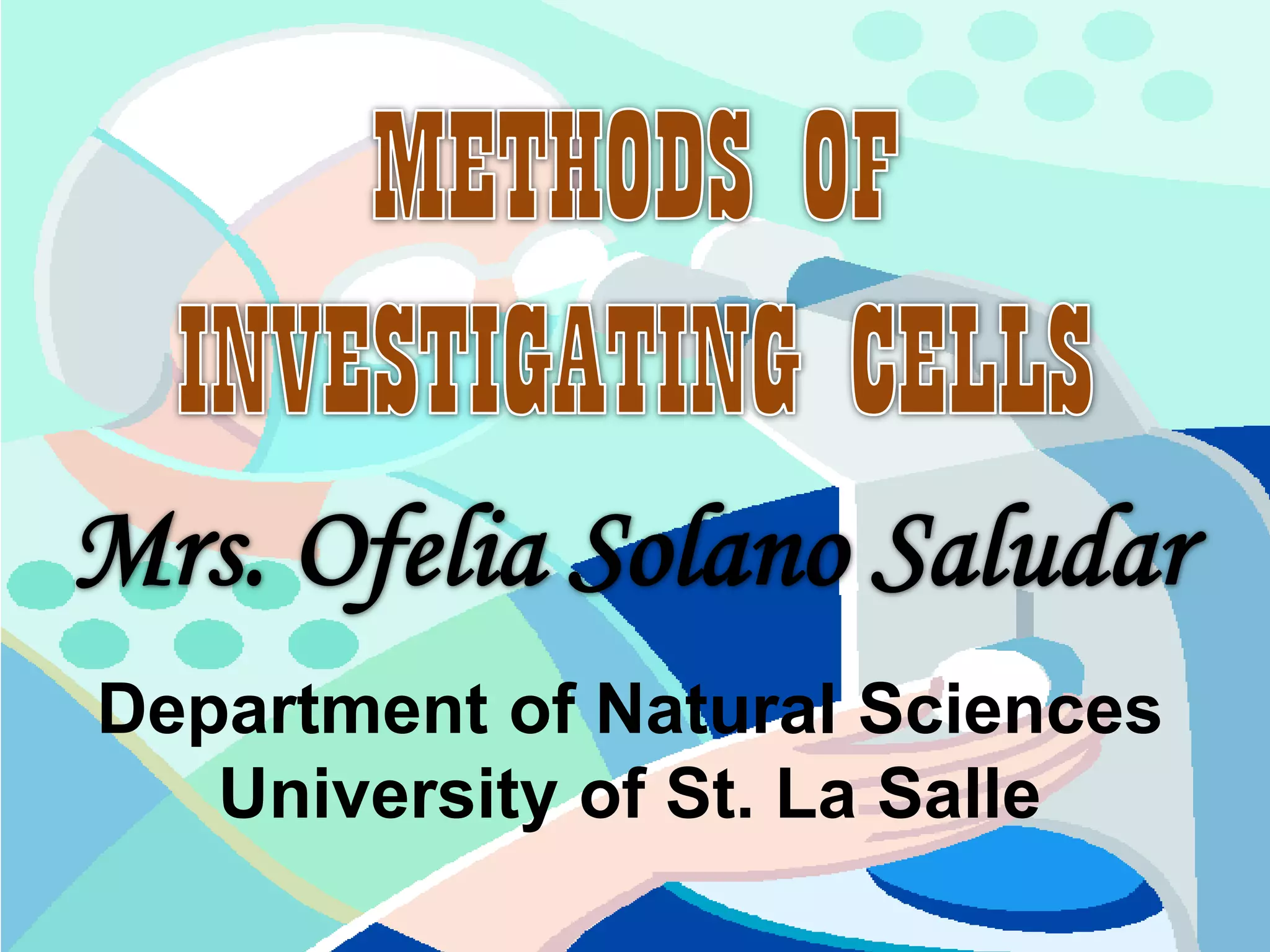
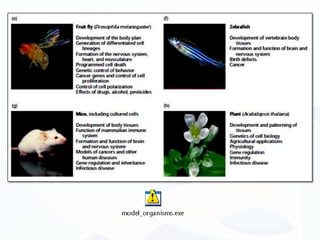
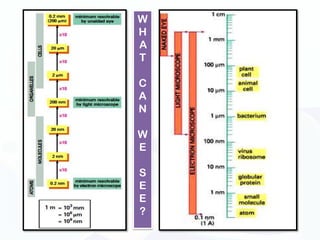
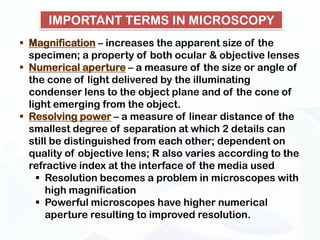
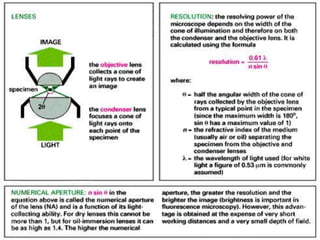
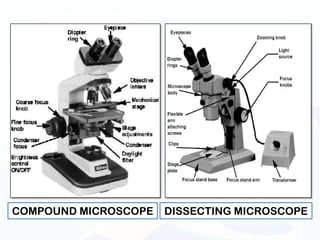
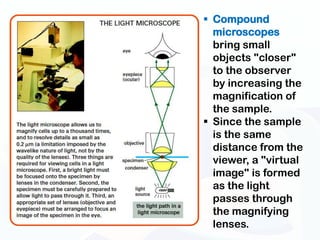
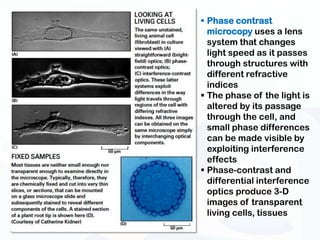
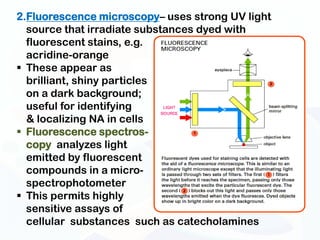
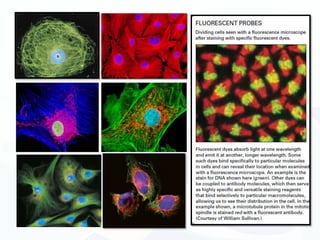
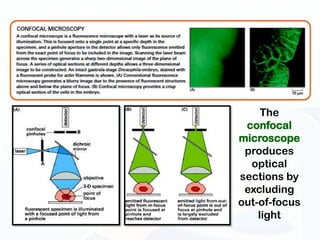
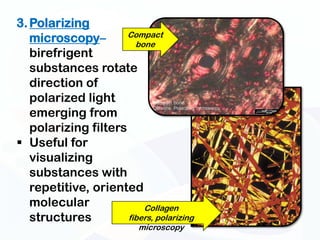
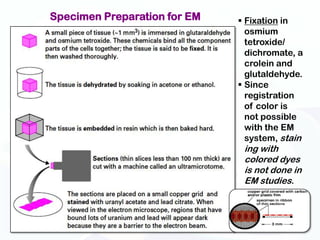
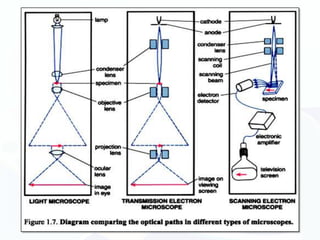
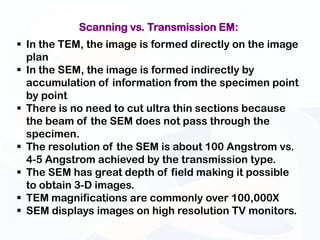
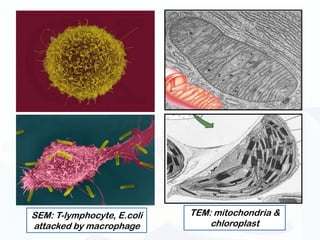
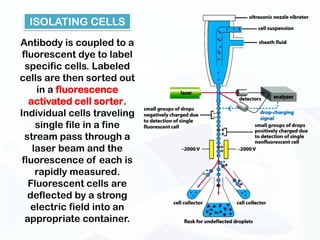
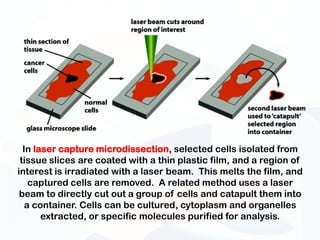
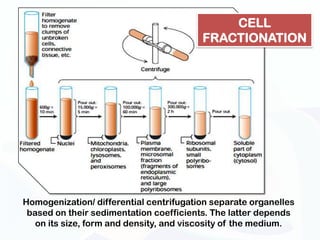
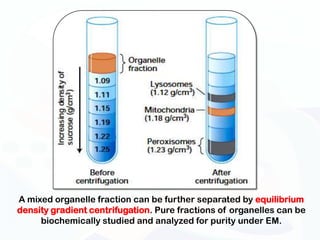
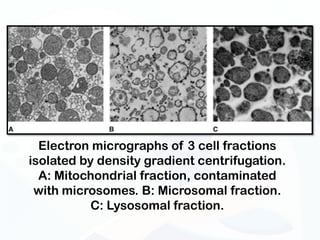
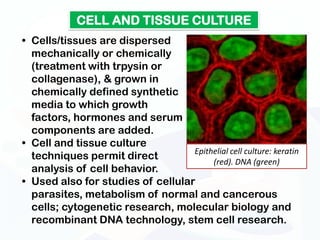
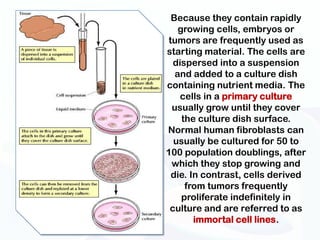
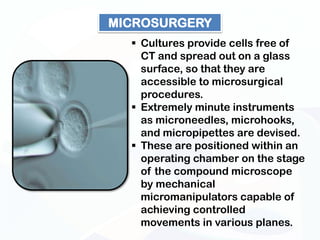
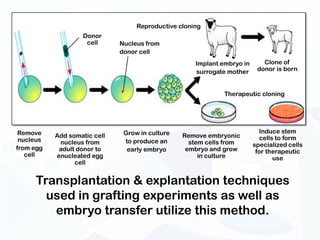
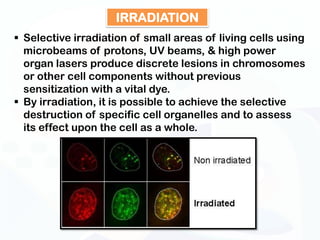
The document discusses various microscopy techniques used to study cells and their parts at the microscopic level. It describes light microscopes like compound, dissecting, and fluorescence microscopes. It also discusses electron microscopes like scanning and transmission electron microscopes. It explains techniques like cell fractionation, cell and tissue culture, laser capture microdissection, and microscopy that allow isolation and study of individual cells and organelles.