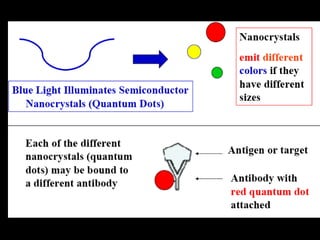
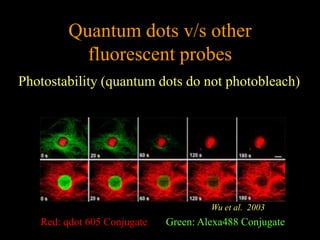
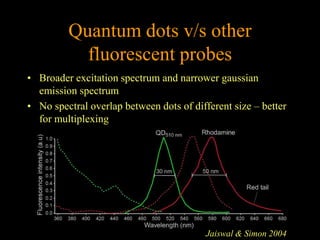
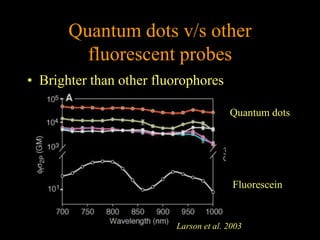
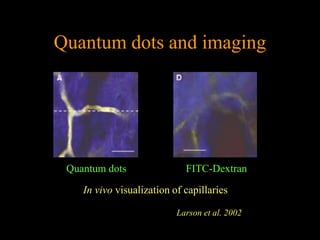
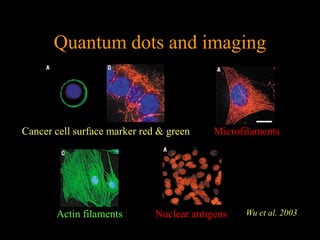
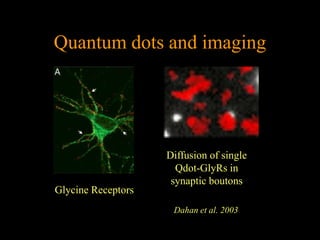
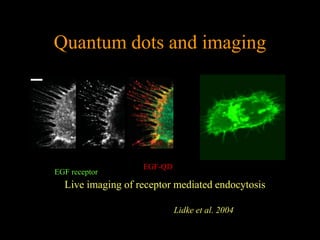
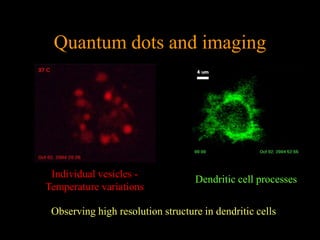
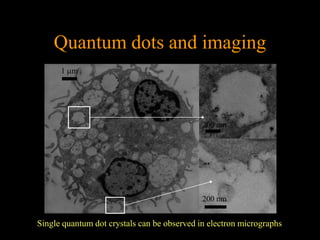
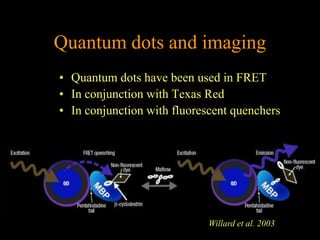
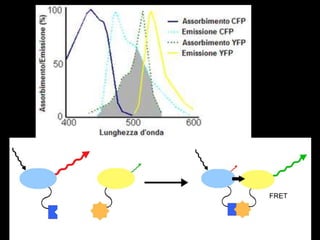
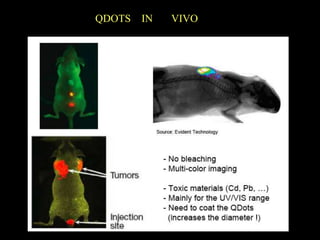
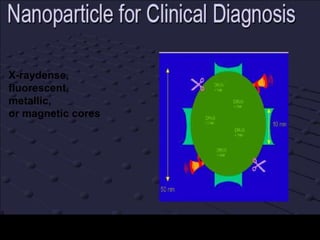
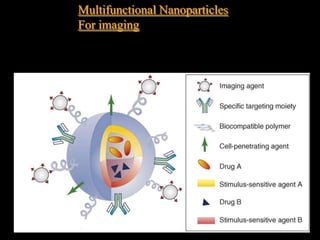
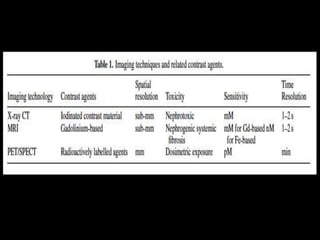
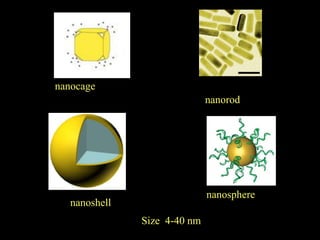
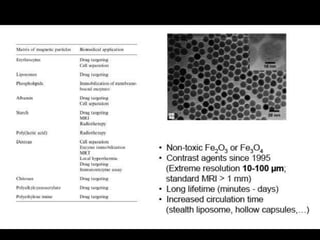
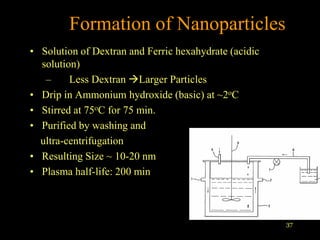
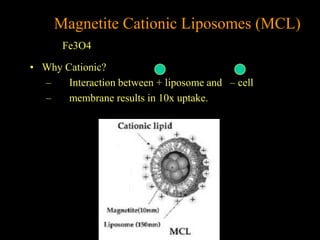
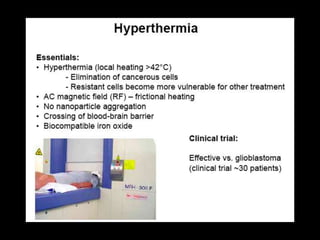
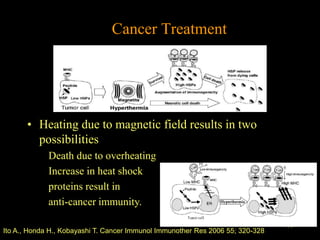
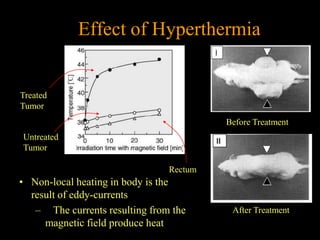
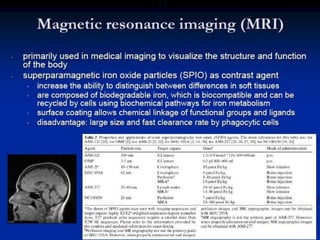
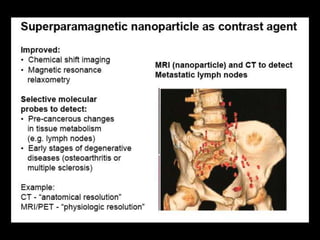
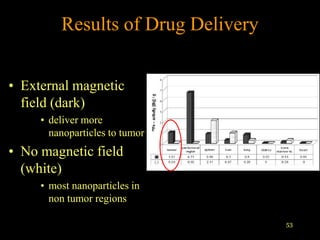
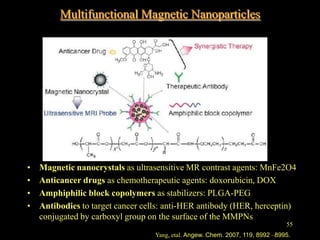
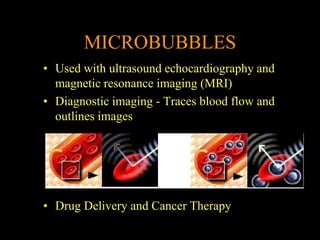
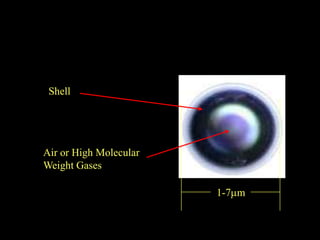
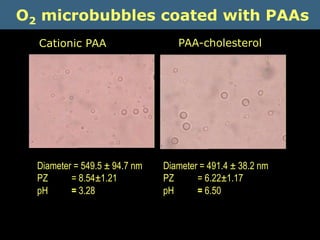
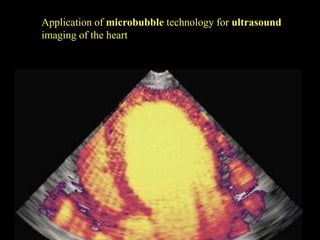
Quantum dots have unique spectral properties that make them useful fluorescent probes for cellular imaging. They can be made water-soluble and conjugated to biomolecules for targeting specific cells and structures. Quantum dots have advantages over traditional fluorescent probes like greater photostability and the ability to multiplex imaging. They have been used for in vitro and in vivo imaging applications like labeling cancer cells, visualizing capillaries and receptors, and observing subcellular structures in real-time. While useful imaging tools, quantum dots have limitations like potential toxicity that must be addressed for in vivo use.