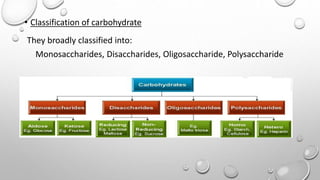
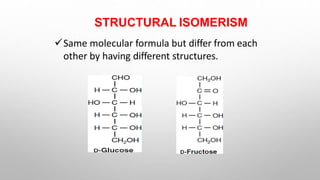
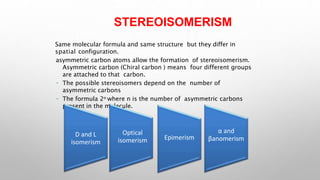
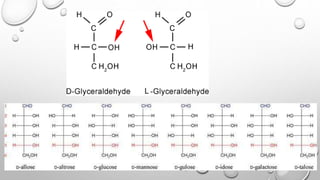
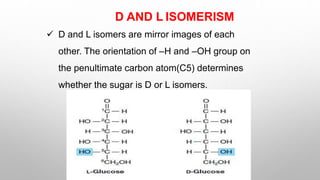
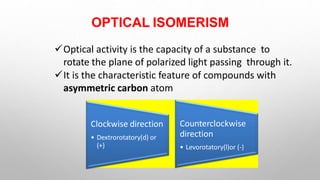
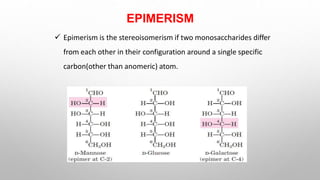
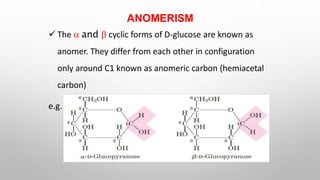
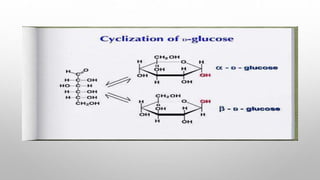
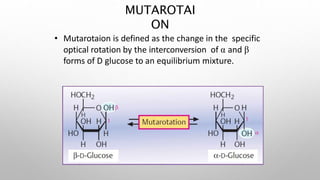
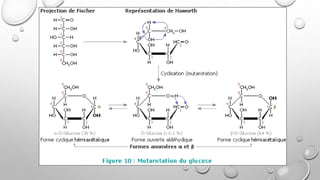
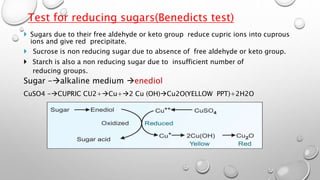
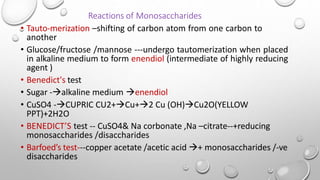
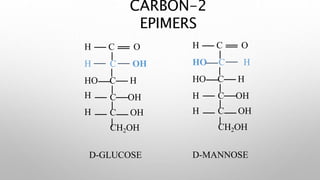
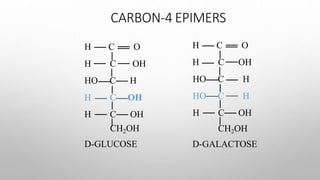
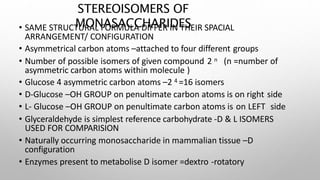
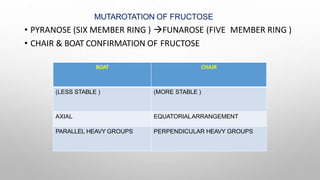
This document discusses the chemistry and functions of carbohydrates. It defines carbohydrates as polyhydroxy aldehydes or ketones and classifies them into monosaccharides, oligosaccharides, and polysaccharides. Monosaccharides include glucose, fructose, and galactose. The document discusses isomerism, mutarotation, epimers, and the reactions of monosaccharides. It describes the roles of carbohydrates as an energy source, precursor for other biomolecules, and structural component in cells. Key monosaccharides like glucose, fructose, and galactose and their importance are highlighted.