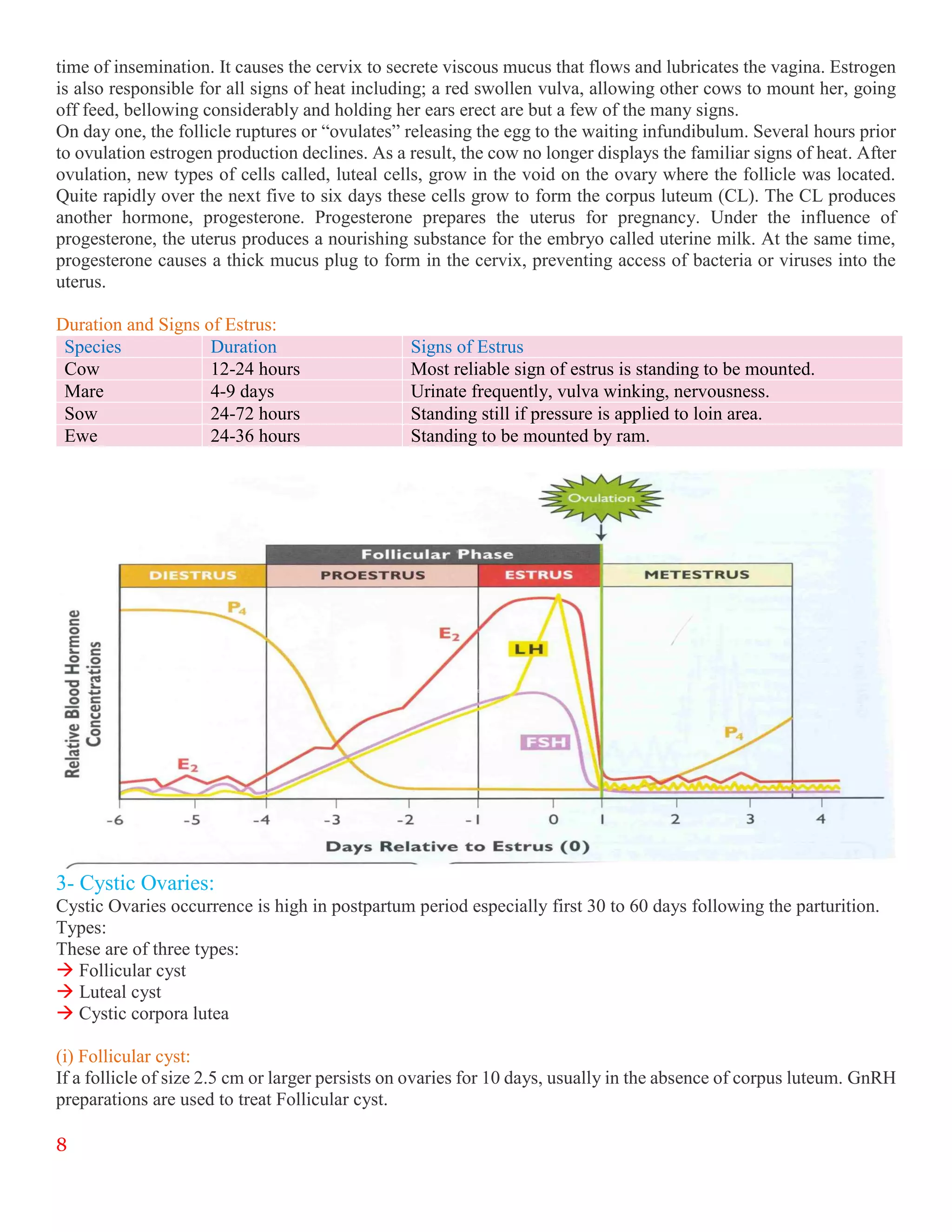
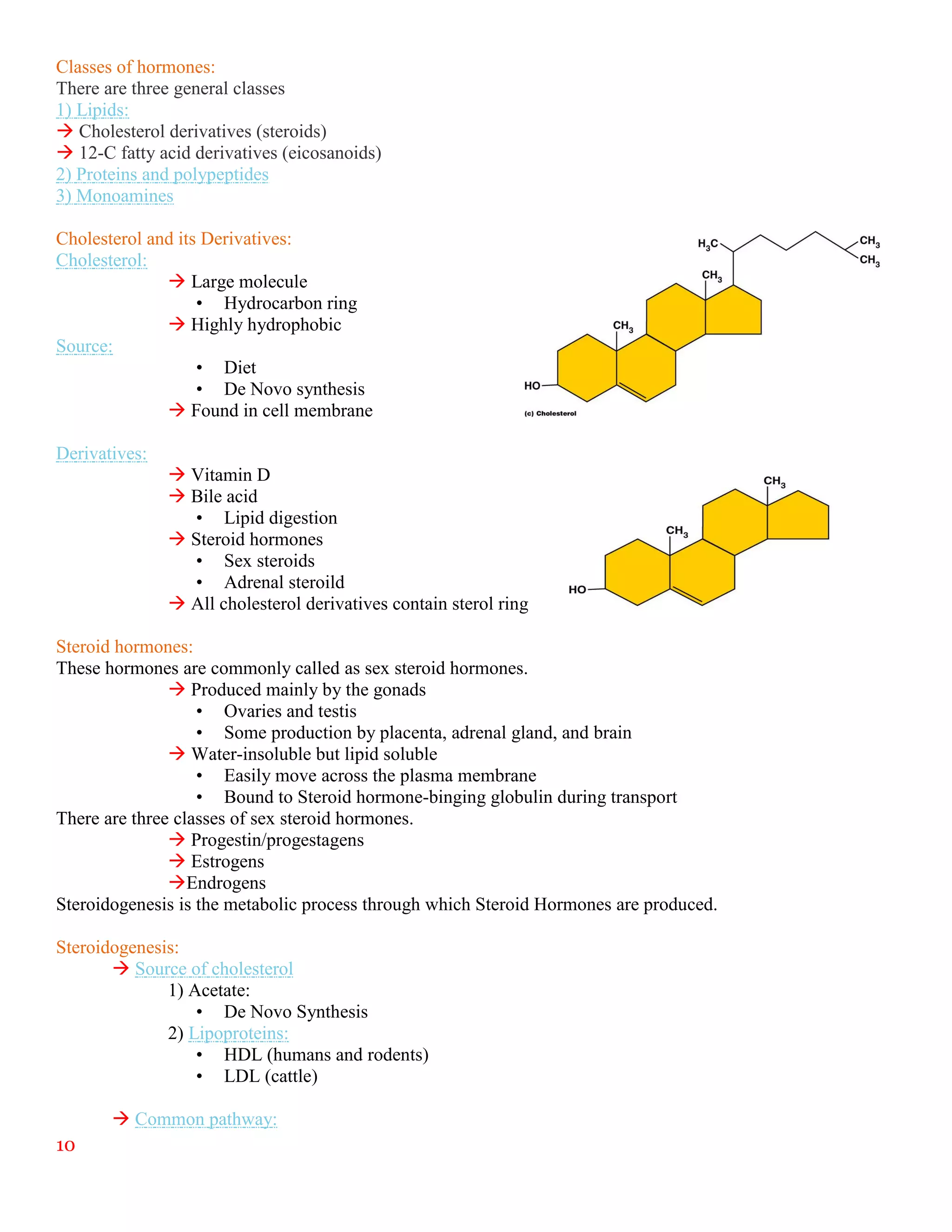
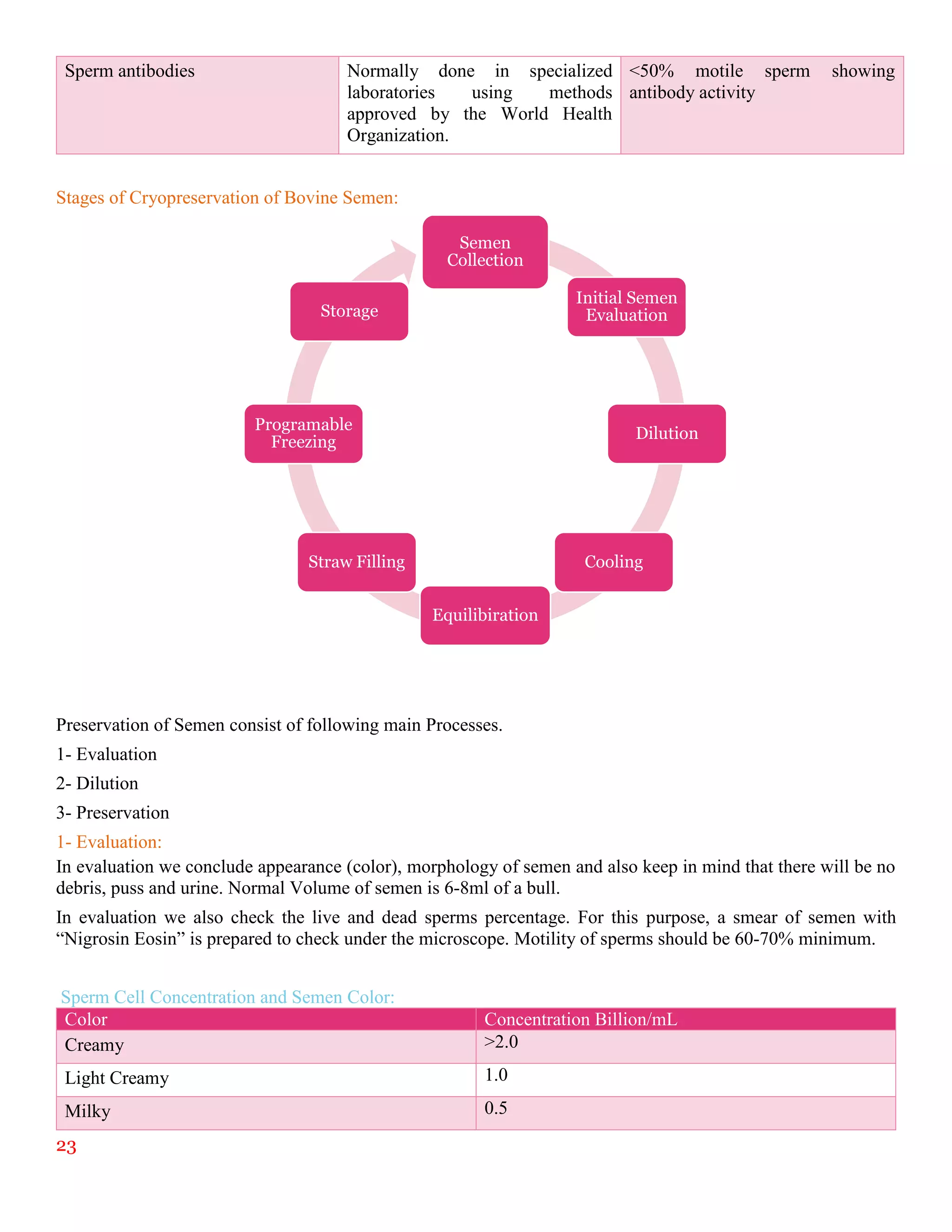
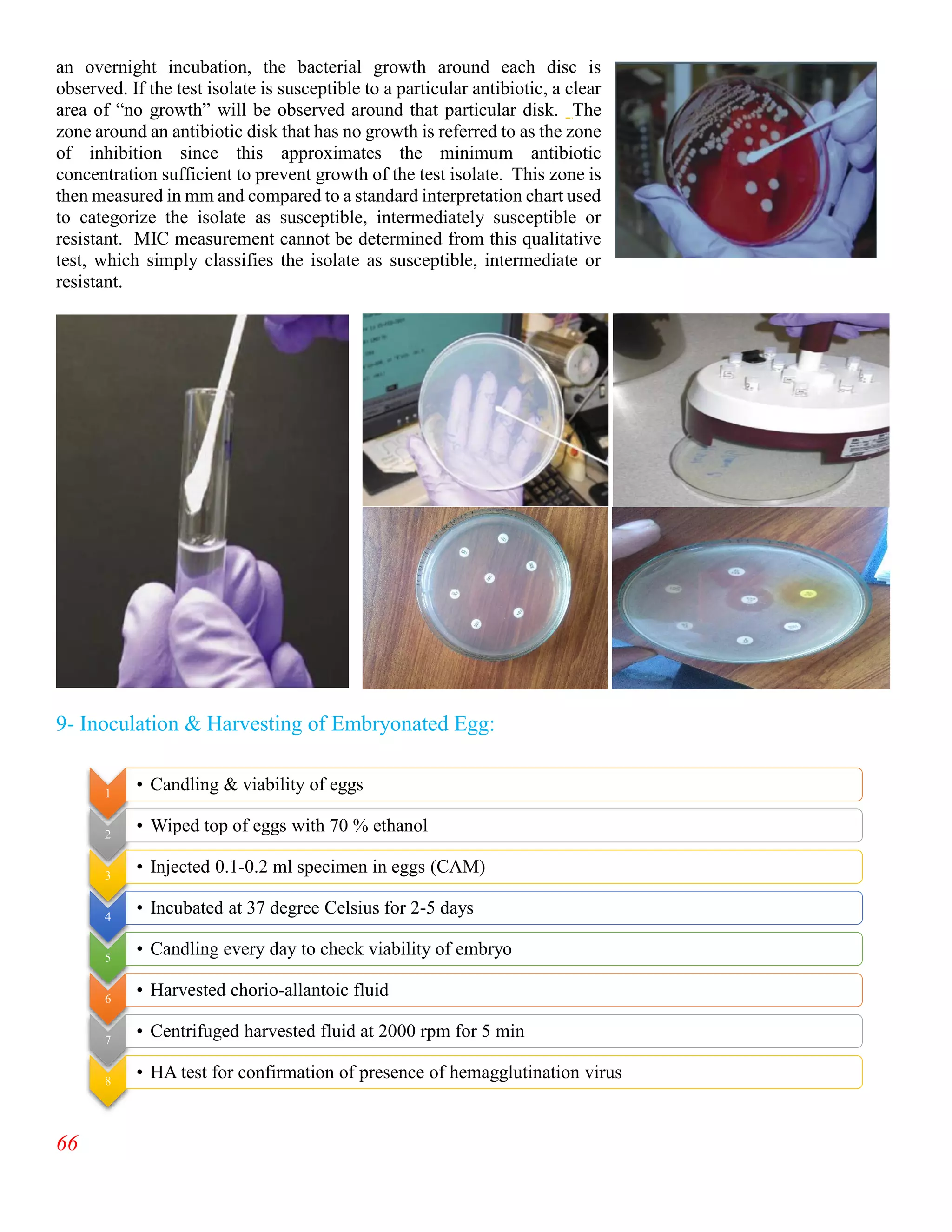
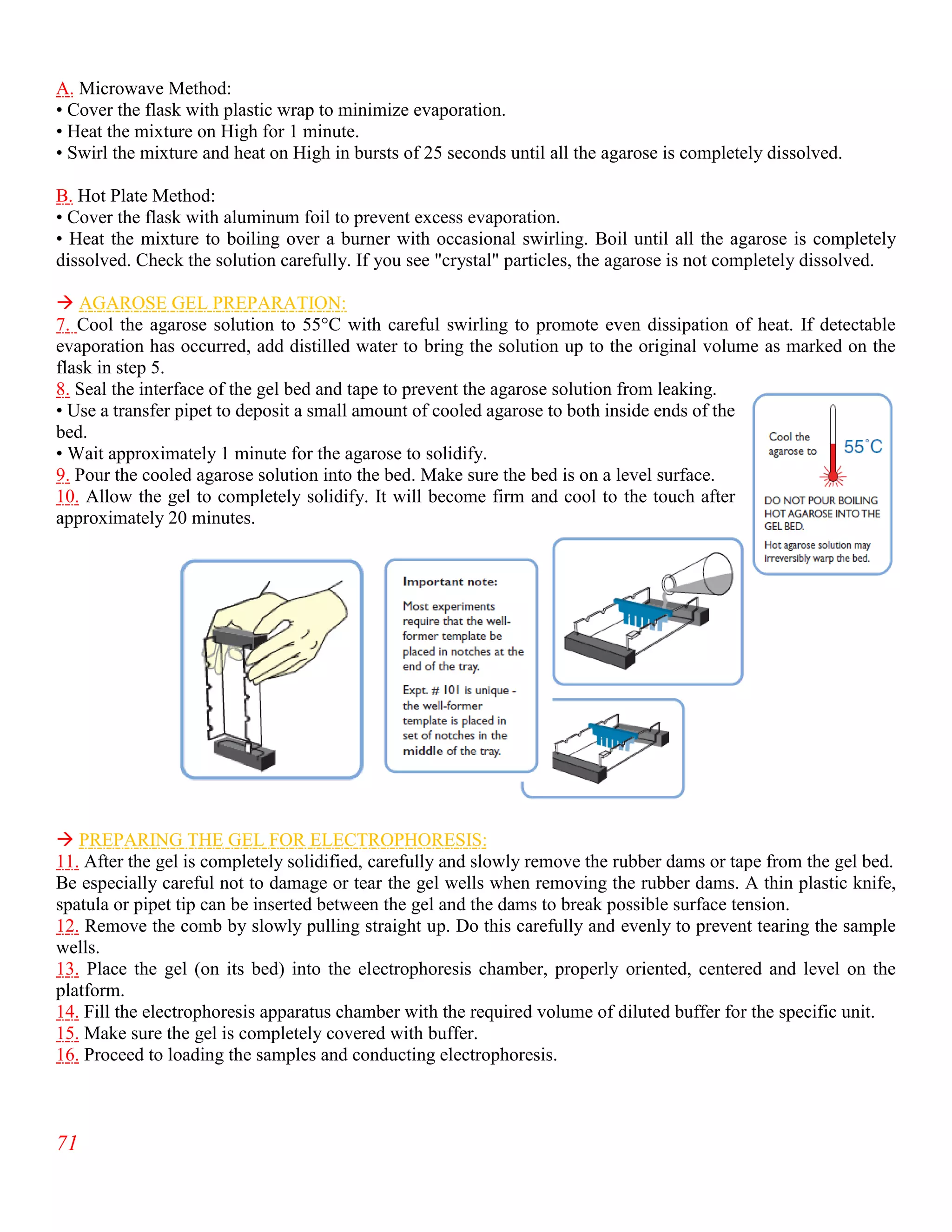
The document summarizes an internship report submitted by two students at the Animal Sciences Institute (ASI) of the National Agricultural Research Council (NARC) in Islamabad, Pakistan. ASI conducts research in animal health, production, and technology. The students completed rotations in eight ASI departments over two months, gaining experience in areas like animal reproduction, livestock, small ruminants, wildlife and poultry, and dairy technology. They studied topics such as bovine reproductive physiology, the estrous cycle, reproductive hormones, and ultrasound use. The report provides an overview of NARC and ASI, and details of the students' work in ASI's Animal Reproduction Section.