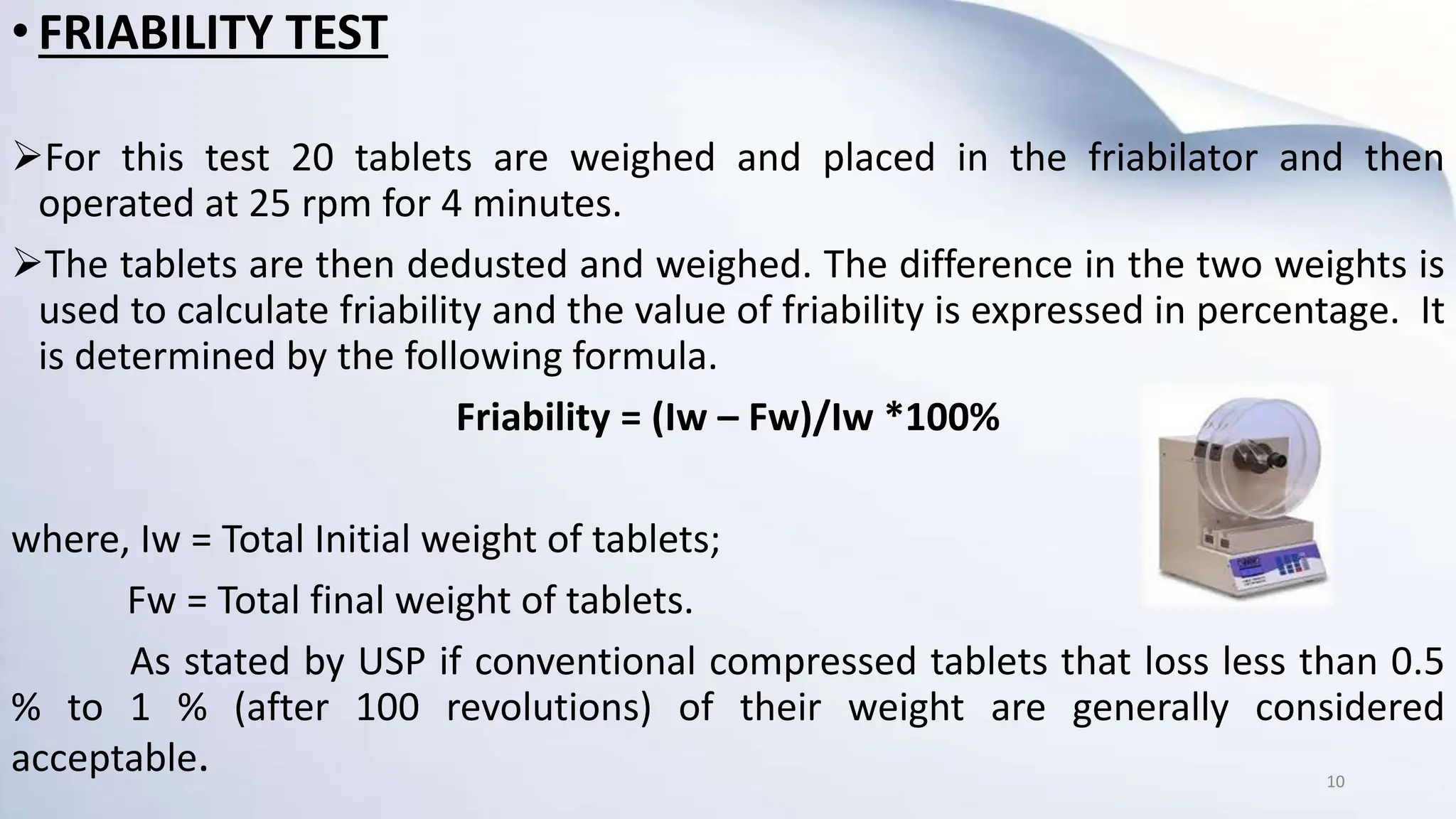
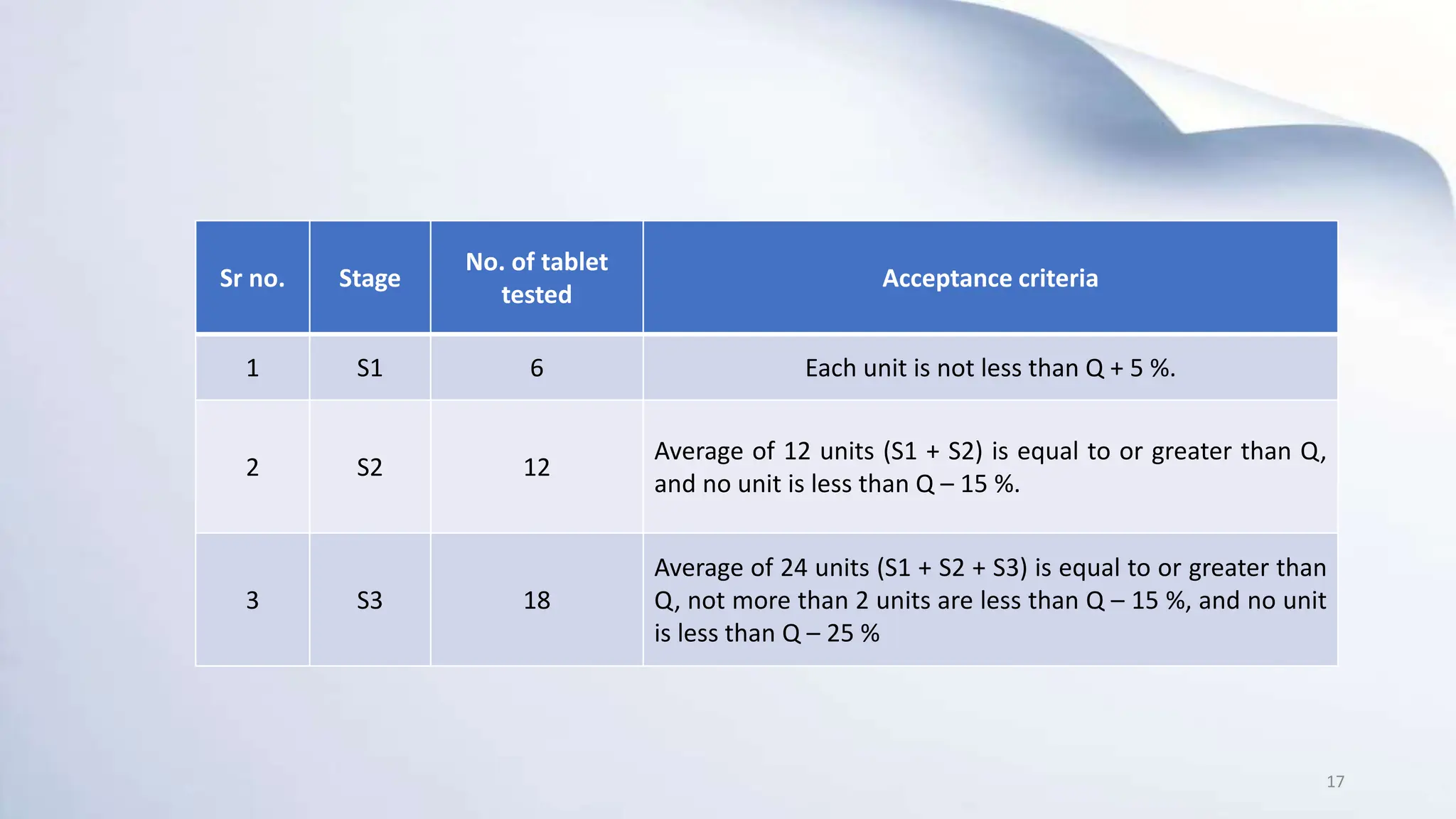
1) In-process quality control (IPQC) tests are tests conducted during the manufacturing process to monitor quality and make adjustments if needed to comply with specifications. Some key IPQC tests for tablets include weight variation, hardness, friability, disintegration, and dissolution.
2) Important reasons for IPQC testing include minimizing human error, providing accurate manufacturing procedures, easier identification of problems, and immediately addressing quality issues. IPQC helps ensure product quality.
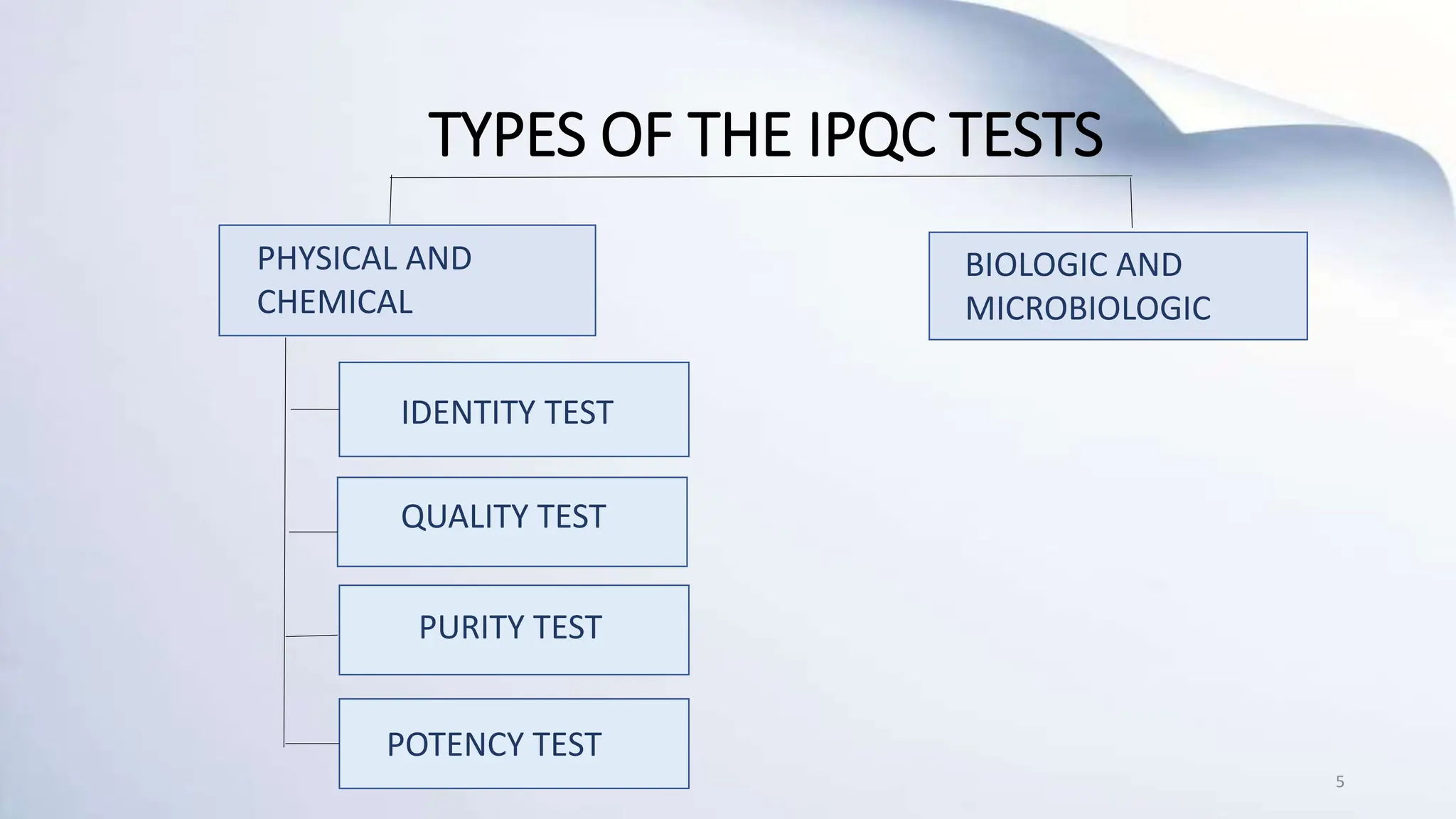
3) Common types of IPQC tests include physical and chemical tests, biological and microbiological tests, and tests for identity, purity, potency, and quality. Official IPQC tests specified for tablets are weight variation, disintegration, dissolution, and drug content