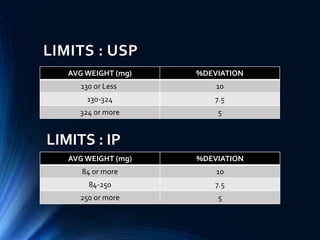
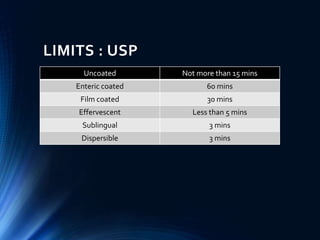
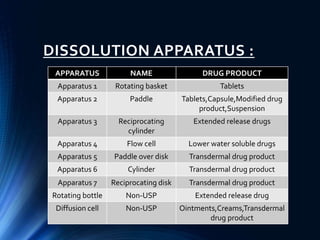
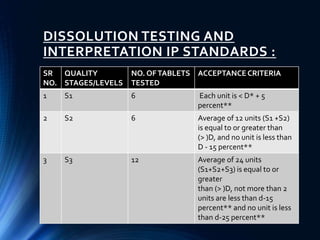
This document summarizes various quality control tests performed on tablets during the manufacturing process, known as in-process quality control (IPQC). It describes tests of tablet thickness, weight variation, hardness, friability, drug content, disintegration time, and dissolution. Acceptance criteria are provided for each test according to pharmacopeial standards. The purpose of IPQC testing is to ensure tablets meet specifications for identity, strength, quality and purity before the manufacturing process is complete.