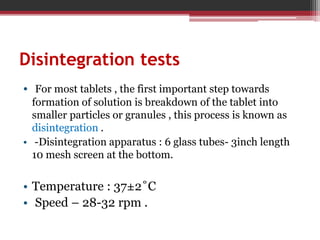
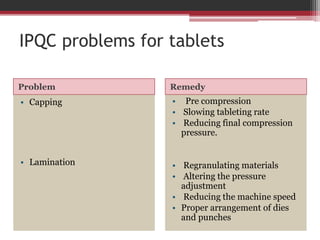
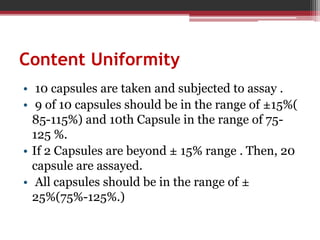
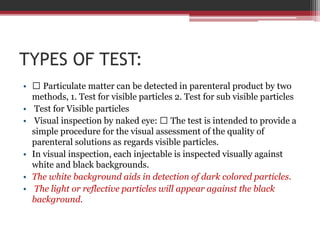
In-process quality control (IPQC) tests are checks carried out during the manufacturing process to monitor and ensure the process complies with specifications. Some key IPQC tests include physical, chemical, microbiological, and biological testing of in-process materials to check for identity, strength, quality, and purity. IPQC tests help minimize errors, provide accurate procedures, identify issues, and ensure proper flow and compliance during production. Common IPQC tests for tablets include weight variation, disintegration, dissolution, drug content, hardness, and friability testing. For suspensions and emulsions, important IPQC tests include checks of appearance, particle size, viscosity, pH, and stability.