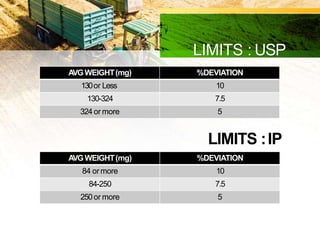
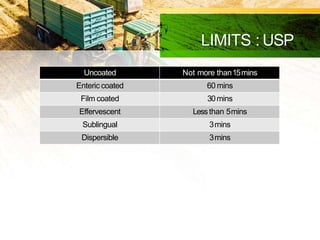
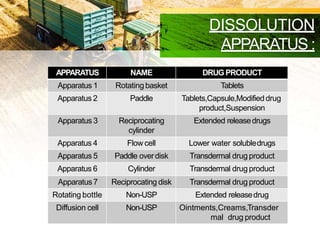
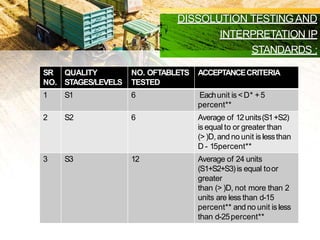
This document discusses in-process quality control tests that are carried out during pharmaceutical manufacturing to ensure quality. It describes tests for general appearance including size, shape, color and odor. It also discusses thickness variation, content uniformity, weight variation, hardness, friability, disintegration time and dissolution tests. Specific test parameters and acceptance criteria are provided for each test to ensure tablets meet standards. In-process quality control helps guarantee that tablets are manufactured correctly and meet specifications before the process is complete.