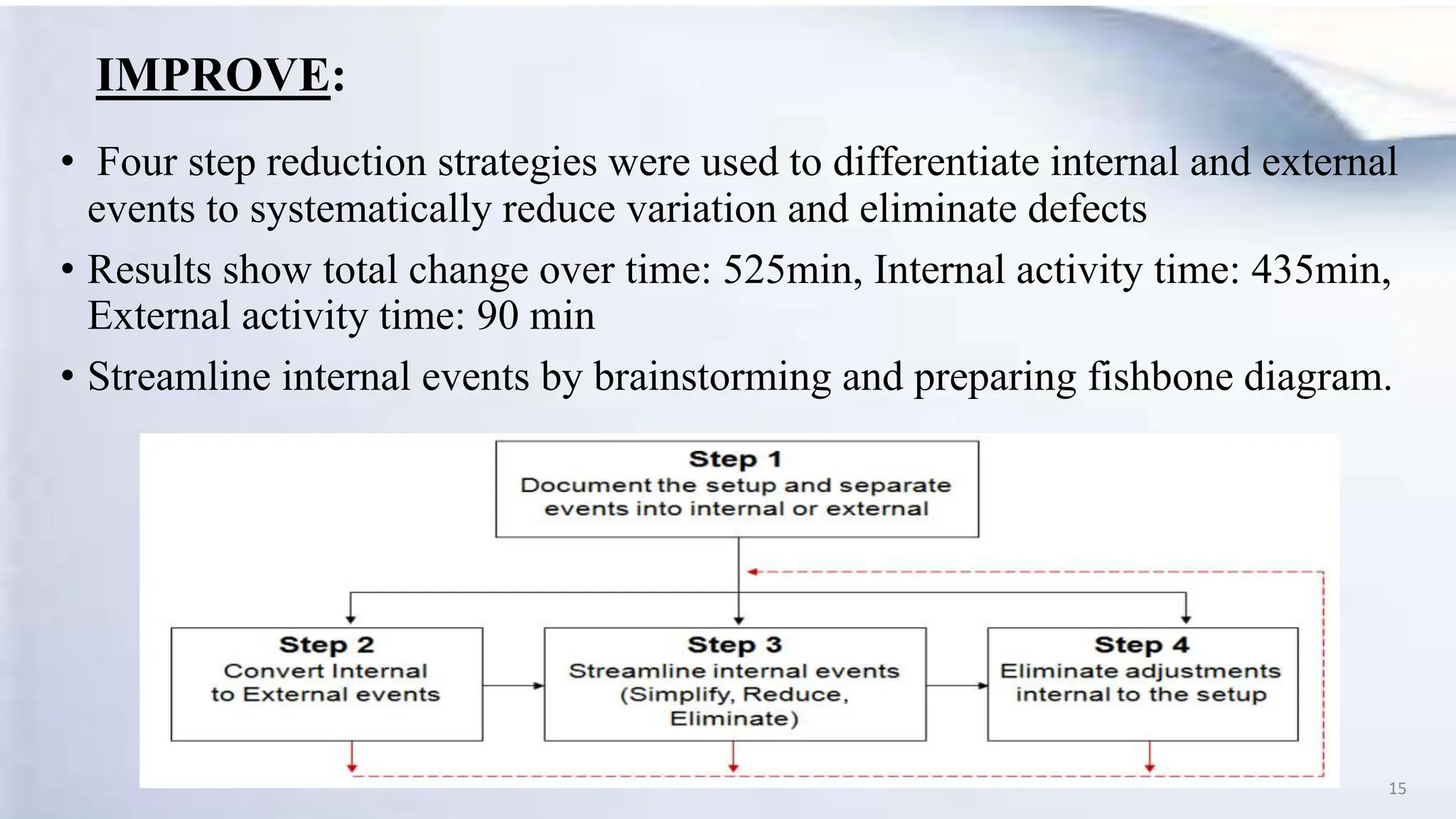
The document provides an overview of Six Sigma, including:
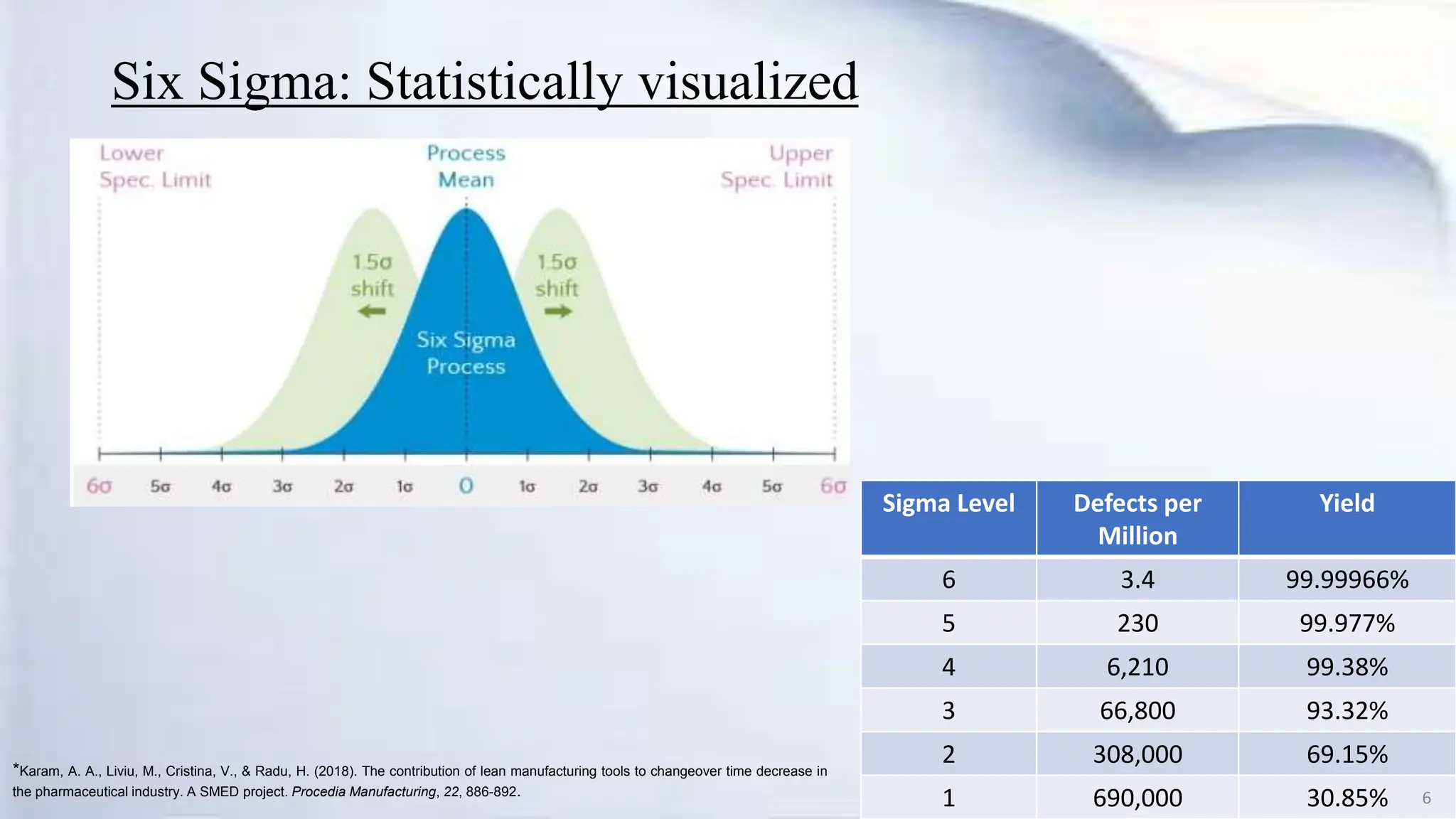
- Six Sigma aims to produce near perfect production processes with only 3.4 defects per million opportunities.
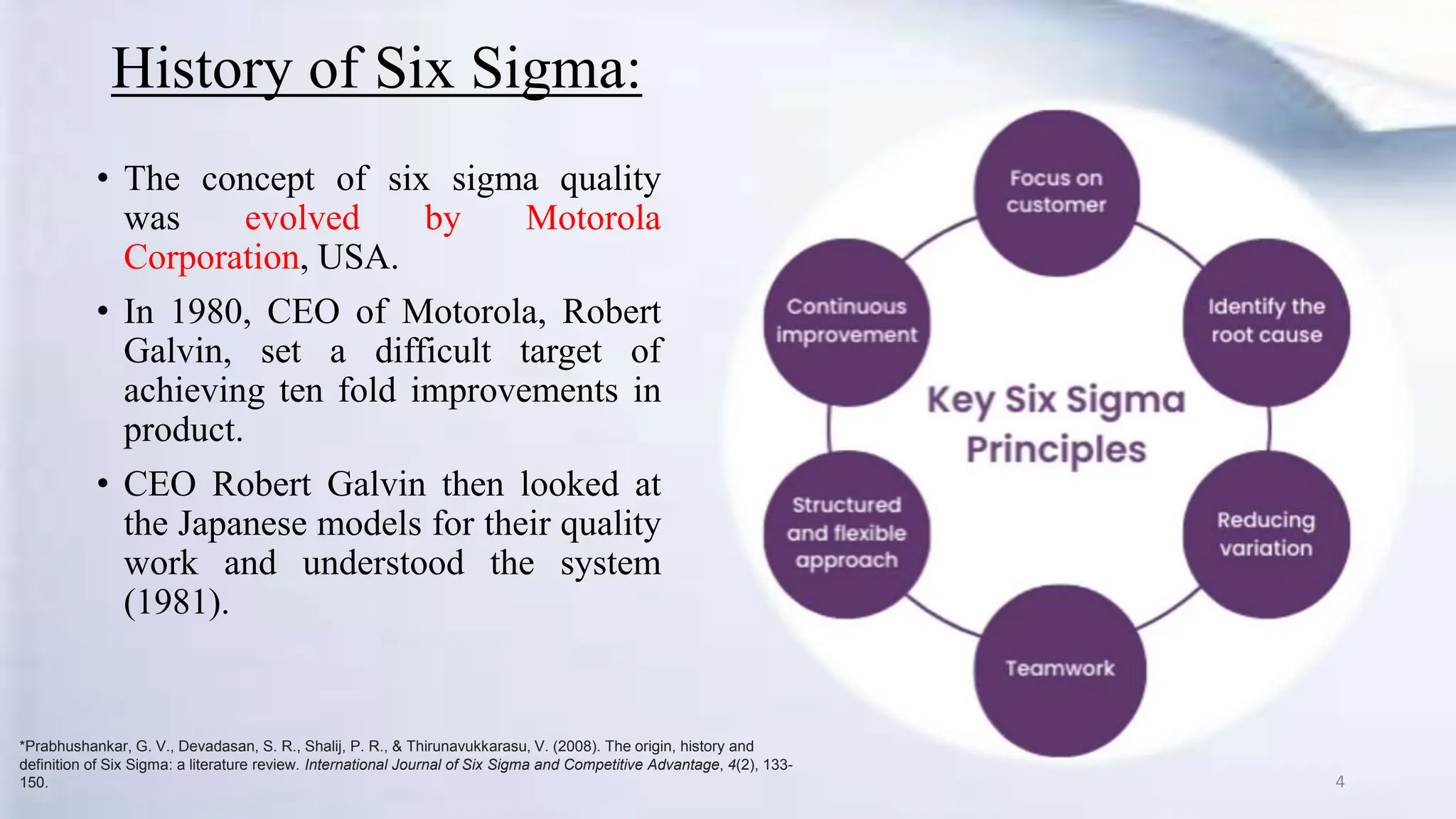
- It was developed by Motorola in the 1980s to achieve dramatic quality improvements.
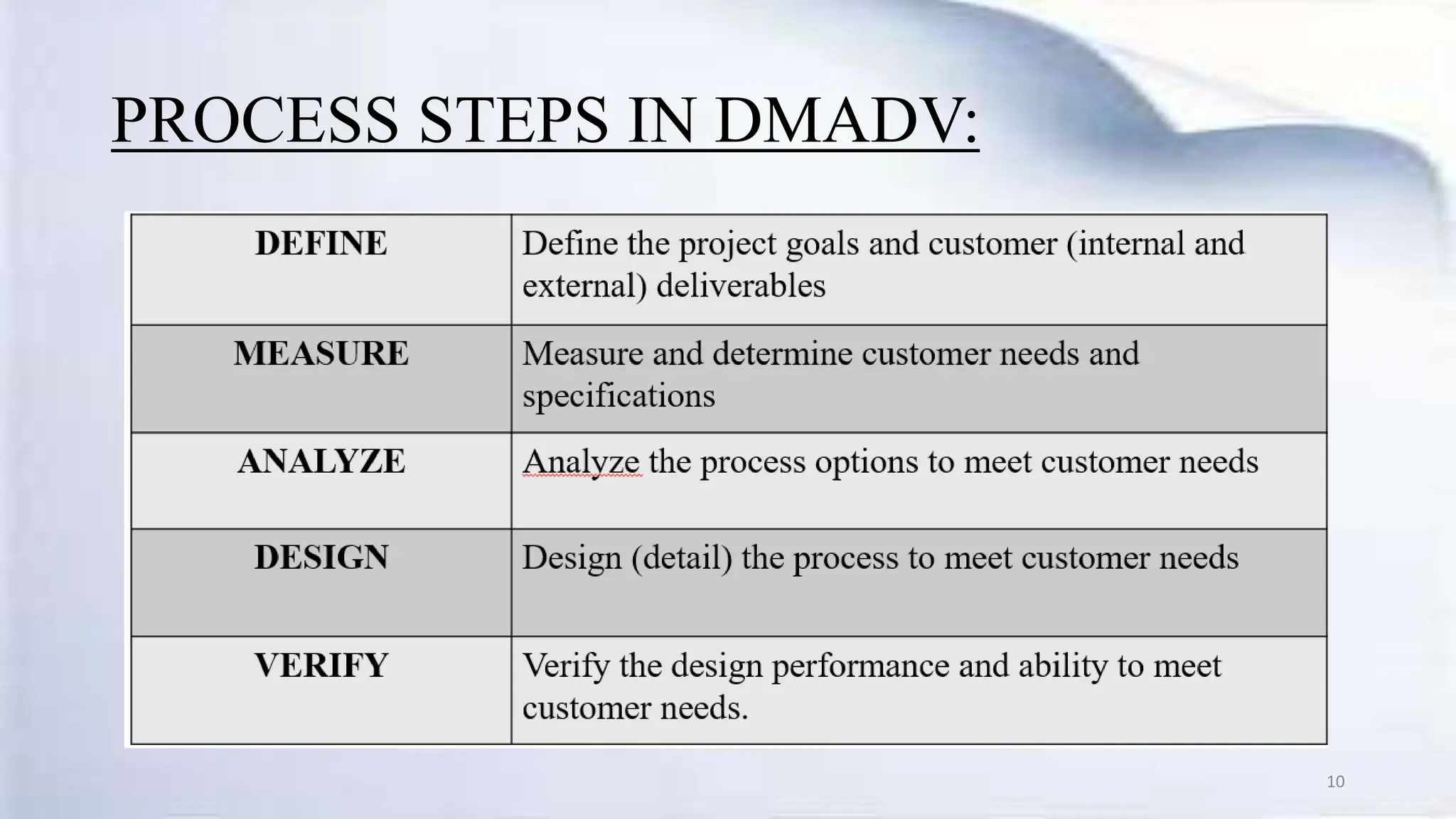
- The DMAIC and DMADV methodologies are used to improve existing and design new processes, following steps like Define, Measure, Analyze, Improve, and Control.
- A case study describes using Six Sigma to reduce the changeover time in a pharmaceutical plant's dry granulation process from 628 minutes to 525 minutes.