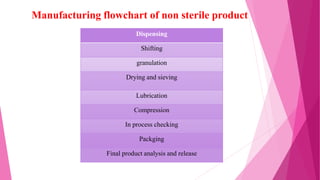
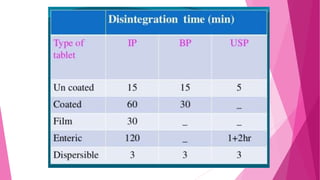
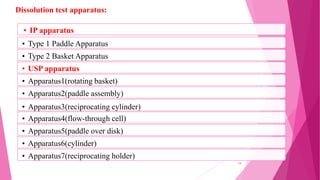
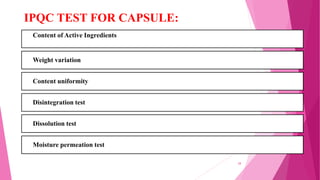
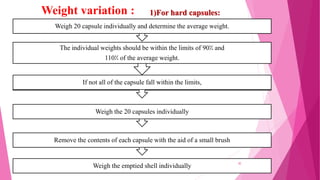
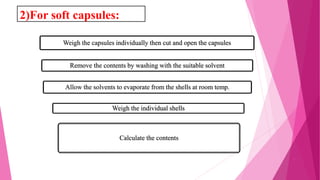
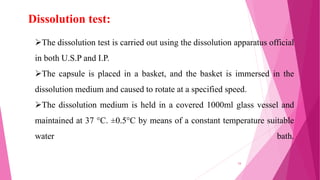
This document provides an overview of in-process quality control (IPQC) tests for non-sterile pharmaceutical tablets and capsules. It discusses the importance of IPQC in minimizing errors and enforcing manufacturing standards. Common IPQC tests described for tablets include weight variation, content uniformity, hardness, thickness, friability, dissolution, disintegration, and moisture content. For capsules, discussed tests include content of active ingredients, weight variation, content uniformity, disintegration, and dissolution. A variety of apparatus used in conducting these tests are also outlined.