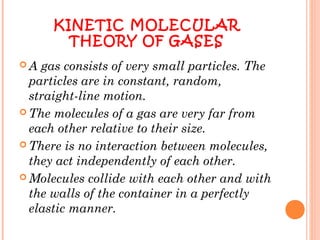
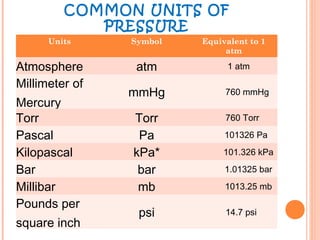
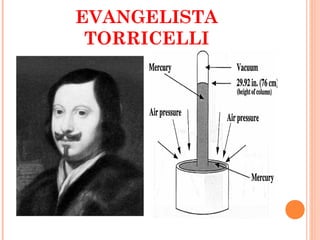
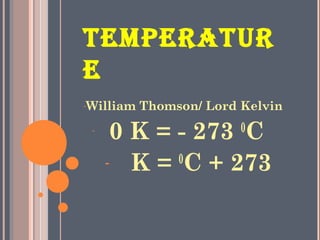
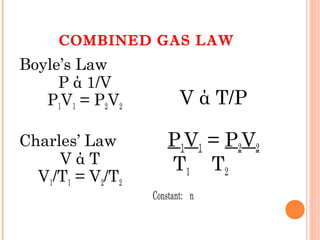
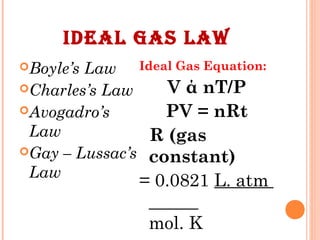
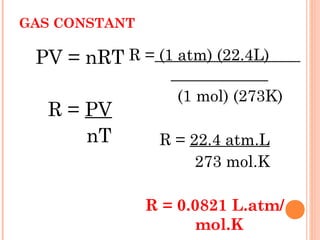
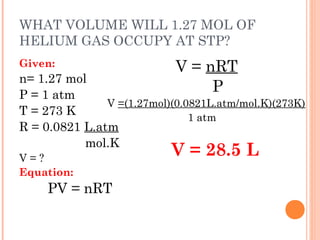
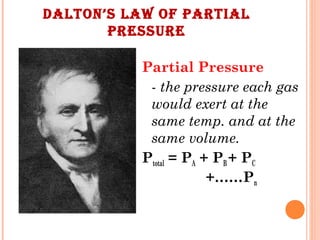
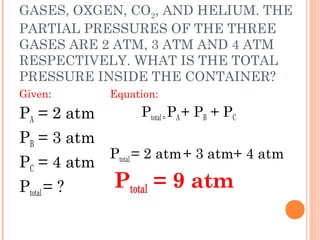
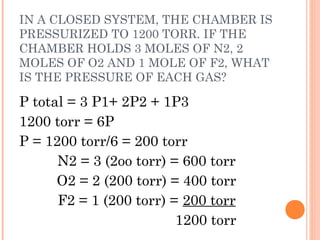
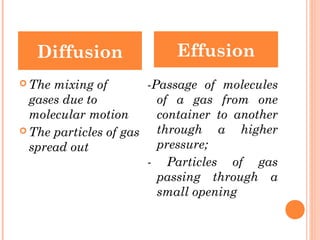
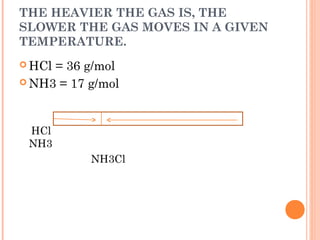
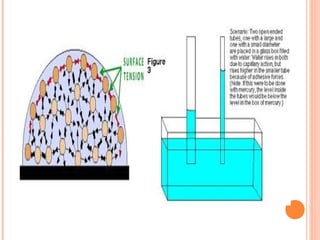
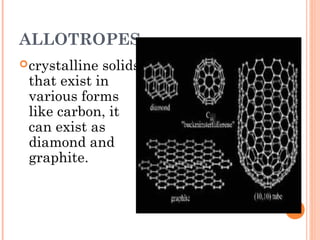
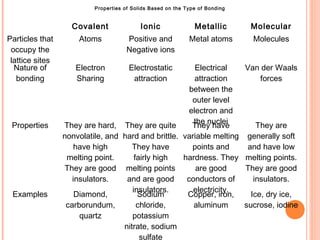
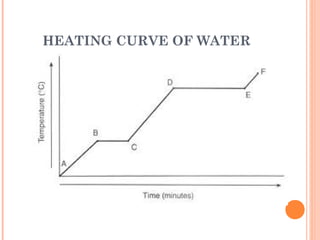
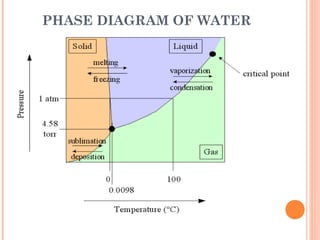
The document discusses the properties and behavior of gases, liquids, and solids from a microscopic perspective. It explains that gases have particles with empty space between them allowing the particles to move freely, while liquids have particles close together allowing them to flow but slide past one another, and solids have particles locked in a fixed structure. The document also covers gas laws, phase changes, types of solids based on bonding, and heating curves. It provides a concise overview of the states of matter and changes between states from a molecular level.