Embed presentation
Downloaded 210 times
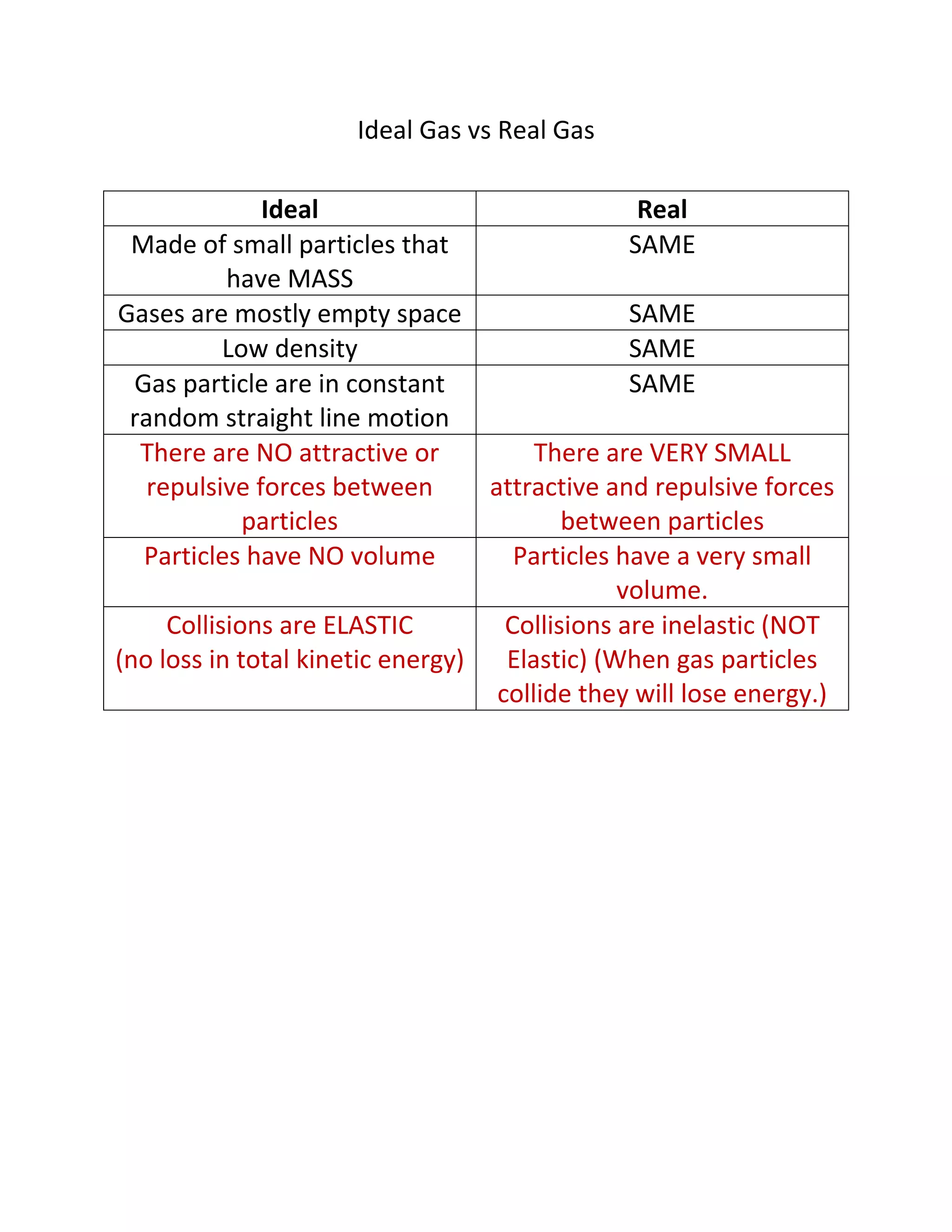
This document compares the key differences between ideal gases and real gases. Ideal gases are made of small particles with mass that have no volume and undergo perfectly elastic collisions with no attractive or repulsive forces. In contrast, real gases have small attractive and repulsive forces between particles, the particles have a small volume, and collisions result in a loss of kinetic energy rather than being perfectly elastic.
