IB Chemistry Real, Ideal Gas and Deviation from Ideal Gas behaviour
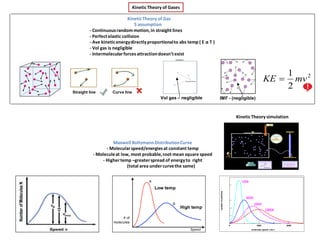
- 1. KineticTheory of Gases Maxwell BoltzmannDistributionCurve - Molecularspeed/energiesat constant temp - Moleculeat low, most probable,root mean square speed - Highertemp –greaterspread of energyto right (total area undercurvethe same) Straight line Curve line Vol gas – negligible IMF - (negligible) Low temp High temp KineticTheory simulation 2 2 1 mvKE KineticTheory of Gas 5 assumption - Continuousrandom motion,in straight lines - Perfectelastic collision - Ave kineticenergydirectlyproportionalto abs temp ( E α T ) - Vol gas is negligible - Intermolecularforcesattractiondoesn’texist
- 2. Kinetic Theory of Gases Distributionof molecularspeed, Xe, Ar, Ne, He at same temp At same temp • Xe, Ar, Ne and He have same Ave KE • Mass He lowest – speed fastest • Mass Xe highest – speed slowest He ArNe Xe Why kineticenergysame for small and large particles?He – mass low ↓ - speed v high ↑ 2 . 2 1 vmKE Xe – mass high ↑ - speed v low ↓ 2 . 2 1 vmKE Kinetic energy SAME Maxwell Boltzman DistributionCurve • Molecularspeed/energy at constant Temp • Moleculeat low, most probableand high speed • Highertemp –greater spread of energyto right • Areaundercurve proportionalto numberof molecules • Wide range of moleculeswith diff KE at particulartemp • Y axis – fractionmoleculeshaving a given KE • X axis – kineticenergy/speedfor molecule 2 2 1 mvKE
- 3. Real Gas vs Ideal Gas Deviation from ideal increase as Moleculeclose together Forcesof attractionexist Kineticenergylow Moleculecloser together– condensed Intermolecularforcesstronger Deviation of Real gas from Ideal No forces attractionForces attraction 1 RT PV PV constant at all pressure, if temp constant 1 RT PV 1 RT PV or Pressure increase ↑ Pressure exert on wall less ↓ 1 RT PV Temp decrease ↓ Temp ↓ PV = nRT Real Gas Vol Intermolecular forces attraction Ideal Gas Vol No intermolecular forces attraction P PV Ideal gas real gas
- 4. At Very High Press Vol gas is significant At Low temp Molecules close together – condense Presence of intermolecular attraction Molecule will exert lower press at wall 1 RT PV Deviation of Real gas from Ideal At High Pressure Molecule close together Forces of attraction exist Vol for molecule to move abt < observed vol because molecule occupy space. As pressure increase, free space formolecule to move become smaller. Vol gas significant at high press Vol used for calculation Actual vol is less for gas to move 1 RT PV Vol used in calculation is vol of container (too large) Click here for notes from chemguide At low temp- greater deviation Negativedeviation Presence of intermolecular attraction Positive deviation Vol of molecules PV = nRT PV P Ideal gas real gas -( ve) deviation (IMF dominates) +(ve) deviation vol dominates
- 5. Boiling point CO2 = - 57oC CH4 = - 1640C N2 = - 195oC H2 = 252oC Deviation greatest for CO2 > CH4 > N2 > H2 Ideal gas eqn At Low pres s+ High Temp Real gas/Van Der Waals eqn Ideal Gas vs Real Gas Ideal gas eqn At High press + Low Temp Real gas/Van Der Waals eqn Correction for press due to IMF bet molecules Correction for vol due to vol occupied by gas molecule Deviation of real gases Temperature decrease ↓ ↓ Higher boiling point ↓ Easier to condense ↓ Intermolecular force increase ↓ Negative deviation PV = nRT PV = nRT Ideal gas Real gas CO2 polar– IMF greater more deviationfrom ideal
- 6. PressureLaw Ideal Gas Equation PV = nRT PV = constant V = constant/P V ∝ 1/p Charles’sLaw PV = nRT 4 diff variables→ P, V, n, T Avogadro’sLaw PV = nRT V = constant x T V = constant T V ∝ T P1V1 = P2V2 V1 = V2 T1 T2 V1 = V2 n1 n2 R = gas constant Unit - 8.314 Jmol-1 K-1 V = Vol gas Unit – m3 PV = nRT Fix 2 variables ↓ changeto diff gas Laws Boyle’sLaw n, T fix n, P fix n, V fix PV = nRT V = constantx n V ∝ n P, T fix P = Pressure Unit – Nm-2 /Pa/kPa n = numberof moles T = Abs Temp in K VolPressure TempVol Temp Pressure Vol n PV = nRT P = constant x T P ∝ T P1 = P2 T1 T2
- 7. Avogadro’s Law Gas Helium Nitrogen Oxygen Mole/mol 1 1 1 Mass/g 4.0 28.0 32.0 Press /atm 1 1 1 Temp/K 273 273 273 Vol/L 22.7L 22.7L 22.7L Particles 6.02 x 1023 6.02 x 1023 6.02 x 1023 22.7L “ equal vol of gases at same temp/press contain equal numbers of molecules” T – 0C (273.15 K) Unit conversion 1 m3 = 103 dm3 = 106 cm3 1 dm3 = 1 litre StandardMolar Volume “molar vol of all gases same at given T and P” ↓ 22.7L 22.7L Video on Avogadro’s Law 1 mole gas • 1 mole of any gas at STP (Std Temp/Press) • occupy a vol of 22.7 dm3 /22 700 cm3 P - 1 atm = 760 mmHg = 100 000 Pa (Nm-2 ) = 100 kPa 22.7L
- 8. Unit conversion 1 atm ↔ 760 mmHg ↔ 100 000 Pa ↔ 100 kPa 1m3 ↔ 103 dm3 ↔ 106 cm3 1 dm3 ↔ 1000 cm3 ↔ 1000 ml ↔ 1 litre x 103 x 103 cm3 dm3 m3 x 10-3 x 10-3 PressureLaw Ideal Gas Equation PV = nRT PV = constant V = constant/P V ∝ 1/p Charles’sLaw Avogadro’sLaw PV = nRT V = constant x T V = constant T V ∝ T P1V1 = P2V2 V1 = V2 T1 T2 V1 = V2 n1 n2 PV = nRT Boyle’sLaw n, T fix n, P fix n, V fix PV = nRT V = constantx n V ∝ n P, T fix PV = nRT P = constant x T P ∝ T P1 = P2 T1 T2 Combined Boyle + Charles + Avogadro 2 22 1 11 T VP T VP Combined Boyle + Charles nRTPV P nT V Find R at molar vol n = 1 mol T = 273K P = 100 000 Pa V = 22.7 x 10-3 m3 R = ? R = 8.31 JK-1 mol-1 nT PV R T = 273K V = 22.7 x 10-3 m3 P = 100 000 Pa
- 9. Volatile Liquid (Propanone) Volatile Gas (Butane) Syringe MethodDirect Weighing Direct Weighing Heated – convert to gas RMM calculated- m, T, P, V, ρ are known n = mass M P RT M P RT V m M RT M m PV nRTPV Density ρ = m (mass) V (vol)PV mRT M RT M m PV nRTPV Molar mass RMM using Ideal Gas Eqn PV = nRT
- 10. DirectWeighing PV = nRT PV = mass x R x T M M = m x R x T PV = 0.52 x 8.314 x 373 101325 x 2.84 x 10-4 = 56.33 1. Cover top with aluminium foil. 2. Make a hole on aluminium foil 3. Record mass flask + foil 4. Pour 2 ml volatile liq to flask 5. Place flask in water, heat to boiling Temp and record press 6. Vapour fill flask when heat 7. Cool flask in ice bath –allow vapour to condense to liquid 8. Take mass flask + foil + liquid Mass flask + foil 115.15 g Mass flask + foil + condensed vapour 115.67 g Mass condensed vapour 0.52 g Pressure 101325 Pa Temp of boiling water 100 0C 373K Vol of flask 284 cm3 2.84 x 10-4 m3 Data Processing Vol gas =Vol water in flask = Mass water Assume density water = 1 g/ml Click here for lab procedure Video on RMM determination RMM (LIQUID) using Ideal Gas Eqn Procedure Data Collection
- 11. DirectWeighing 1. Fill flask with water and invert it . 2. Record press + temp of water 3. Mass of butane + lighter (ini) 4. Release gas into flask 6. Measure vol gas 7. Mass of butane + lighter (final) Total Press (atm) = partial P(butane) + partial P(H2O) P butane = P(atm) – P(H2O) = (760 – 19.32) mmHg P butane = 743.911 mmHg → 99.17Pa Dalton’s Law of Partial Press: Total press of mix of gas = sum of partial press of all individual gas 5. Adjust water level in flask until the same as atm pressure RMM butane RMM butane Collection gas RMM (GAS) using Ideal Gas Eqn Procedure Data Collection Mass butane + lighter 87.63 g Mass butane + lighter (final) 86.98 g Mass butane 0.65 g Pressure 99.17 Pa Temp of boiling water 21.7 0C 294 K Vol of flask 276 cm3 2.76 x 10-4 m3 Data Processing PV = nRT PV = mass x R x T M M = m x R x T PV = 0.65 x 8.314 x 294 99.17 x 2.76 x 10-4 = 58.17
- 12. SyringeMethod 1. Set temp furnace to 98C. 2.Put 0.2ml liq into a syringe 3. Record mass syringe + liq 5. Inject liq into syringe 6. Liq will vaporise , Record vol of heat vapour + air 4. Record vol of heated air. Mass syringe + liq bef injection 15.39 g Mass syringe + liq after injection 15.27 g Mass of vapour 0.12 g Pressure 100792Pa Temp of vapour 371 K Vol heated air 7 cm3 Vol heated air + vapour 79 cm3 Vol of vapour 72 – 7 = 72 cm3 7.2 x 10-5 m3 Video on RMM determination RMM (LIQUID)using Ideal Gas Eqn Data CollectionProcedure Data Processing PV = nRT PV = mass x R x T M M = m x R x T PV = 0.12 x 8.314 x 371 100792 x 7.2 x 10-5 = 51.1
- 13. P = 101 kNm-2 = 101 x 103 Nm-2 Calculate RMM of gas Mass empty flask = 25.385 g Mass flask fill gas = 26.017 g Mass flask fill water = 231.985 g Temp = 32C, P = 101 kPa Find molar mass gas by direct weighing, T-23C , P- 97.7 kPa Mass empty flask = 183.257 g Mass flask + gas = 187.942 g Mass flask + water = 987.560 g Mass gas = (187.942 – 183.257) = 4.685 g Vol gas = Vol water = Mass water = (987.560 – 183.257) = 804.303 cm3 RMM determination PV = nRT PV = mass x R x T M M = mass x R x T PV = 4.685 x 8.314 x 296 97700 x 804.303 x 10-6 = 146.7 Vol gas = 804.303 cm3 = 804.303 x 10-6 m3 P = 97.7 kPa = 97700 Pa Density water = 1g/cm3 M = m x RT PV = 0.632 x 8.314 x 305 101 x 103 x 206 x 10-6 = 76.8 m gas = (26.017 – 25.385) = 0.632 g vol gas = (231.985 – 25.385) = 206 x 10-6 m3 X containC, H and O. 0.06234 g of X combusted, 0.1755 g of CO2 and0.07187 g of H2O produced. Find EF of X Element C H O Step 1 Mass/g 0.0479 0.00805 0.006384 RAM/RMM 12 1 16 Step 2 Number moles/mol 0.0479/1 2 = 0.00393 0.00805/1 = 0.00797 0.006384/16 = 0.000393 Step 3 Simplest ratio 0.00393 0.000393 = 10 0.00797 0.000393 = 20 0.000393 0.000393 = 1 Conservation of mass Mass C atom before = Mass C atom after Mass H atom before = Mass C atom after CHO + O2 CO2 + H2O Mol C atom in CO2 = 0.1755 = 0.00393 mol 44 Mass C = mol x RAM C = 0.00393 x 12 = 0.0479 g Mol H atom in H2O = 0.07187 = 0.0039 x 2 = 0.00797 mol 18 Mass H = mol x RAM H = 0.00797 x 1.01 = 0.00805 g Mass of O = (Mass CHO – Mass C – Mass H) = 0.06234 – 0.0479 - 0.00805 = 0.006384 g 0.06234 g 0.1755 g 0.07187 g Empirical formula – C10H20O1
- 14. Find EF for X with composition by mass. S 23.7 %, O 23.7 %, CI 52.6 % Given, T- 70 C, P- 98 kNm-2 density - 4.67g/dm3 What molecular formula? Empirical formula - SO2CI2 Density ρ = m (mass) V (vol) Element S O CI Composition 23.7 23.7 52.6 Moles 23.7 32.1 = 0.738 23.7 16.0 = 1.48 52.6 35.5 = 1.48 Mole ratio 0.738 0.738 1 1.48 0.738 2 1.48 0.738 2 P RT M P RT V m M RT M m PV nRTPV Density = 4.67 gdm-3 = 4.67 x 10-3 gm-3 M = (4.67 x 10-3) x 8.31 x (273 +70) 9.8 x 104 M = 135.8 135.8 = n [ 32 + (2 x 16)+(2 x 35.5) ] 135.8 = n [ 135.8] n = 1 MF = SO2CI2 P = 98 kN-2 = 9.8 x 104 Nm-2 3.376 g gas occupies 2.368 dm3 at T- 17.6C, P - 96.73 kPa. Find molar mass PV = nRT PV = mass x RT M M = mass x R x T PV = 3.376 x 8.314 x 290.6 96730 x 2.368 x 10-3 = 35.61 Vol = 2.368 dm3 = 2.368 x 10-3 m3 P – 96.73 kPa → 96730Pa T – 290.6K 6.32 g gas occupy 2200 cm3, T- 100C , P -101 kPa. Calculate RMM of gas PV = nRT n = PV RT n = (101 x 103) (2200 x 10-6) 8.31 x ( 373 ) n = 7.17 x 10-2 mol Vol = 2200 cm3 = 2200 x 10-6 m3 RMM = mass n RMM = 6.32 7.17 x 10-2 = 88.15
- 15. Sodiumazide, undergoesdecompositionrxn to produceN2 used in air bag 2NaN3(s) → 2Na(s) + 3N2(g) Temp, mass and pressure was collected in table below i. State number of sig figures for Temp, Mass, and Pressure i. Temp – 4 sig fig Mass – 3 sig fig Pressure – 3 sig fig Temp/C Mass NaN3/kg Pressure/atm 25.00 0.0650 1.08 ii. Find amt, mol of NaN3 present ii. iii. Find vol of N2, dm3 produced in these condition RMM NaN3 – 65.02 molMol RMM mass Mol 00.1 02.60 0.65 P nRT V nRTPV n = 1.50 mol P – 1.08 x 101000 Pa = 109080 Pa 2NaN3(s) → 2Na(s) + 3N2(g) T – 25.00 + 273.15 = 298.15K 2 mol – 3 mol N2 1 mol – 1.5 mol N2 33 1.340341.0 109080 15.29831.850.1 dmmV V P nRT V Densitygas is 2.6 gdm-3 , T- 25C , P – 101 kPa Find RMM of gas P RT M P RT V m M RT M m PV nRTPV Density ρ = m (mass) V (vol) M = (2.6 x 103) x 8.31 x (298) 101 x 103 M = 63.7
- 16. Sodiumazide, undergoesdecompositionrxn to produceN2 used in air bag 2NaN3(s) → 2Na(s) + 3N2(g) Temp, mass and pressure was collected in table below Temp/C Volume N2/L Pressure/atm 26.0 36 1.15 Find mass of NaN3 needed to produce 36L of N2 RMM NaN3 – 65.02 RT PV n nRTPV 1.1 x 65.02 = 72 g NaN3 P – 1.15 x 101000 Pa = 116150 Pa 2NaN3(s) → 2Na(s) + 3N2(g) T – 26.0 + 273.15 = 299.15K 3 mol N2 – 2 mol NaN3 1.7 mol N2 – 1.1 mol NaN3 moln n 7.1 15.29931.8 1036116150 3 Vol = 36 dm3 = 36 x 10-3 m3 Convert mole NaN3 → Mass /g Densitygas is 1.25g dm-3 at T- 25C ,P- 101 kPa. Find RMM of gas P RT M P RT V m M RT M m PV nRTPV Density ρ = m (mass) V (vol) M = (1.25 x 103) x 8.31 x (298) 101 x 103 M = 30.6
- 17. PV mRT M RT M m PV nRTPV Copper carbonate, CuCO3, undergo decomposition to produce a gas. Determine molar mass for gas X CuCO3(s) → CuO(s) + X (g) Temp, mass, vol and pressure was collected in table below Temp/K Vol gas/ cm3 Pressure/kPa Mass gas/g 293 38.1 101.3 0.088 Find Molar mass for gas X P – 101300 Pa T – 293 K Vol = 38.1 cm3 = 38.1 x 10-6 m3 5.55 101.38101300 29331.8088.0 6 M M Potassium chlorate, KCIO3, undergo decomposition to produce a O2. Find amt O2 collected and mass of KCIO3 decomposed KCIO3 Temp/K Vol gas/ dm3 Pressure/kPa 299 0.250 101.3 2KCIO3(s) → 2KCI(s) + 3O2 (g) RT PV n nRTPV 2 3 .010.0 29931.8 10250.0101300 Omoln n Vol = 0.250 dm3 = 0.250 x 10-3 m3 P – 101300 Pa Convert mole KCIO3 → Mass 2KCIO3 → 2KCI+ 3O2 2 mol – 3 mol O2 0.0066 mol – 0.01 mol O2 0.0066 x 122.6 = 0.81 g KCIO3 RMM KCIO3 – 122.6
