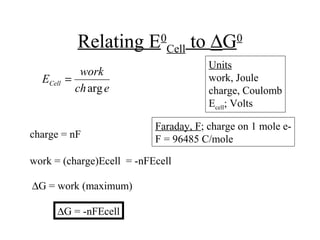
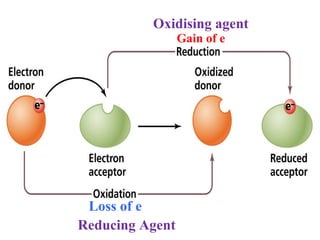
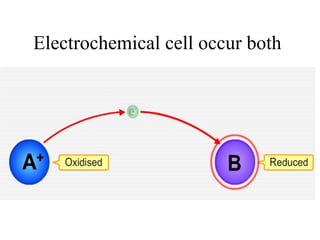
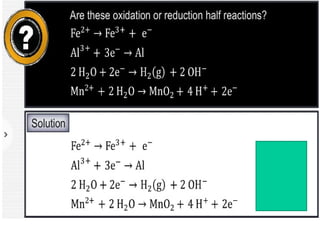
The document discusses redox reactions and electrochemistry, detailing the types of electrochemical cells, such as galvanic and electrolytic cells, along with their operations and examples. It covers concepts like conductance, cell potential, standard reduction potentials, and the relationship between electrochemical parameters and spontaneity of reactions. Additionally, it highlights practical applications, including batteries and fuel cells, underscoring their functioning principles.

































































![19.2
Cell Notation
Zn (s) + Cu2+
(aq) Cu (s) + Zn2+
(aq)
[Cu2+
] = 1 M & [Zn2+
] = 1 M
Zn (s) | Zn2+
(1 M) || Cu2+
(1 M) | Cu (s)
anode cathode
Zn (s)| Zn+2
(aq, 1M)| K(NO3) (saturated)|Cu+2
(aq, 1M)|Cu(s)
anode cathodeSalt bridge
More detail..](https://image.slidesharecdn.com/electrochemistry12-151027130043-lva1-app6892/85/Electrochemistry-12-70-320.jpg)