Embed presentation
Downloaded 14 times

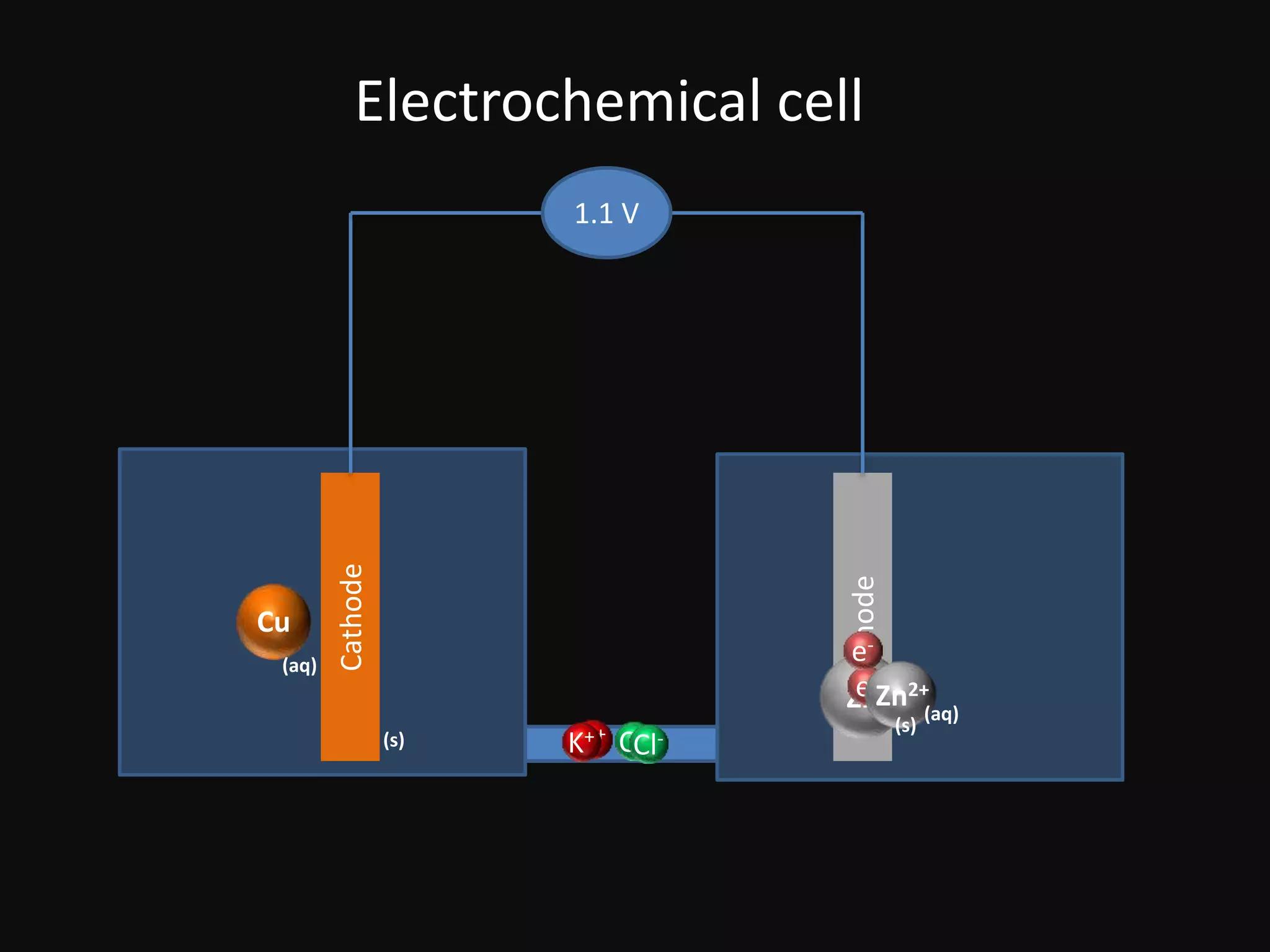


This document discusses single electrode potential and standard electrode potential. Single electrode potential measures an element's tendency to lose or gain electrons when in its own solution, representing the individual electrode's contribution to cell voltage. Standard electrode potential is the potential of a cell where the right electrode is a redox couple and the left electrode is a standard hydrogen electrode, with all species at unit activity and 298K temperature.




Discussion on single electrode potential and standard electrode potential in electrochemistry.
Diagrams of electrochemical cells illustrating cathode and anode components, with a voltage of 1.1 V.
Explanation of single electrode potential as the tendency to gain/lose electrons and standard electrode potential at unit activity and conditions.