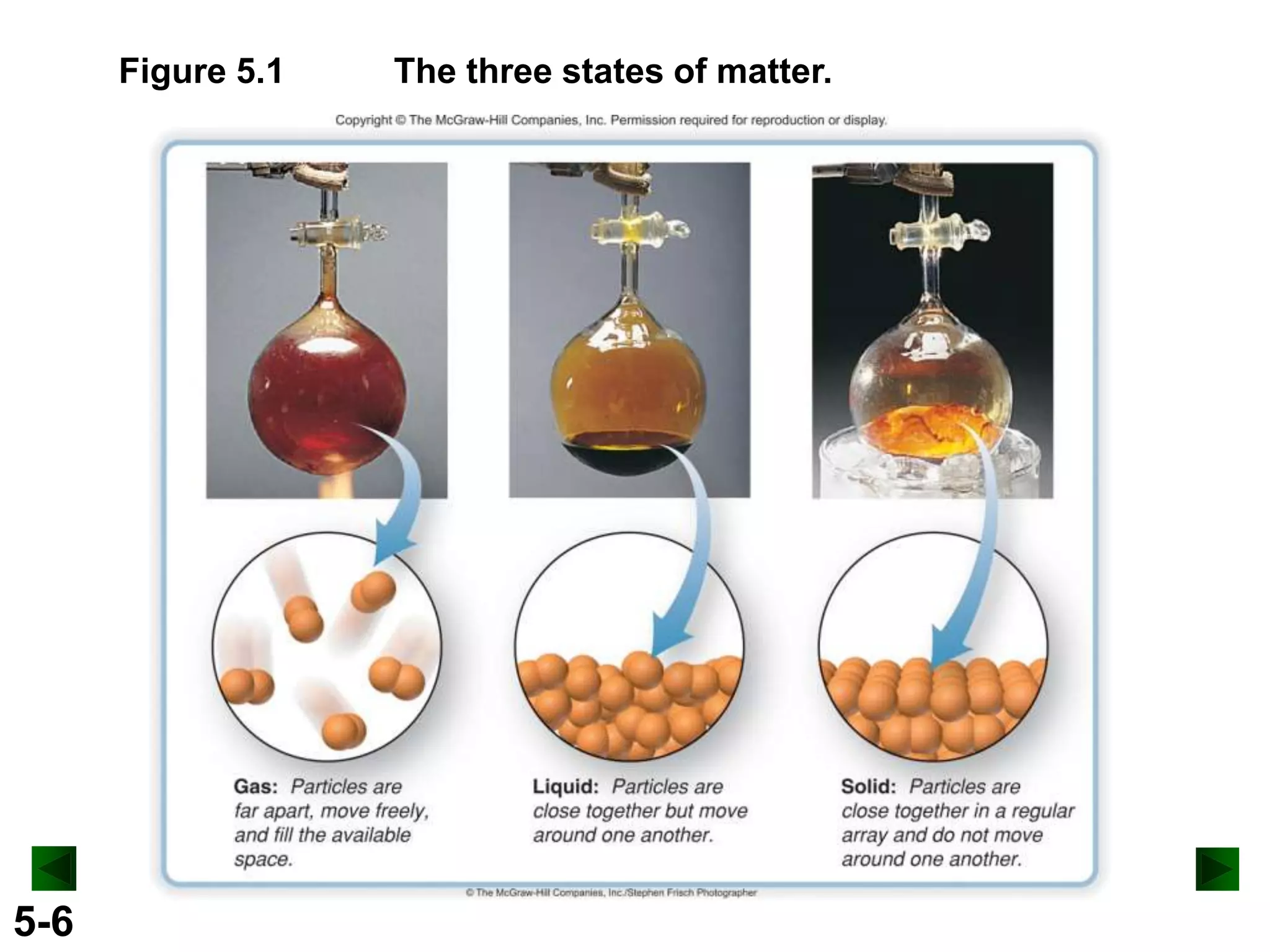
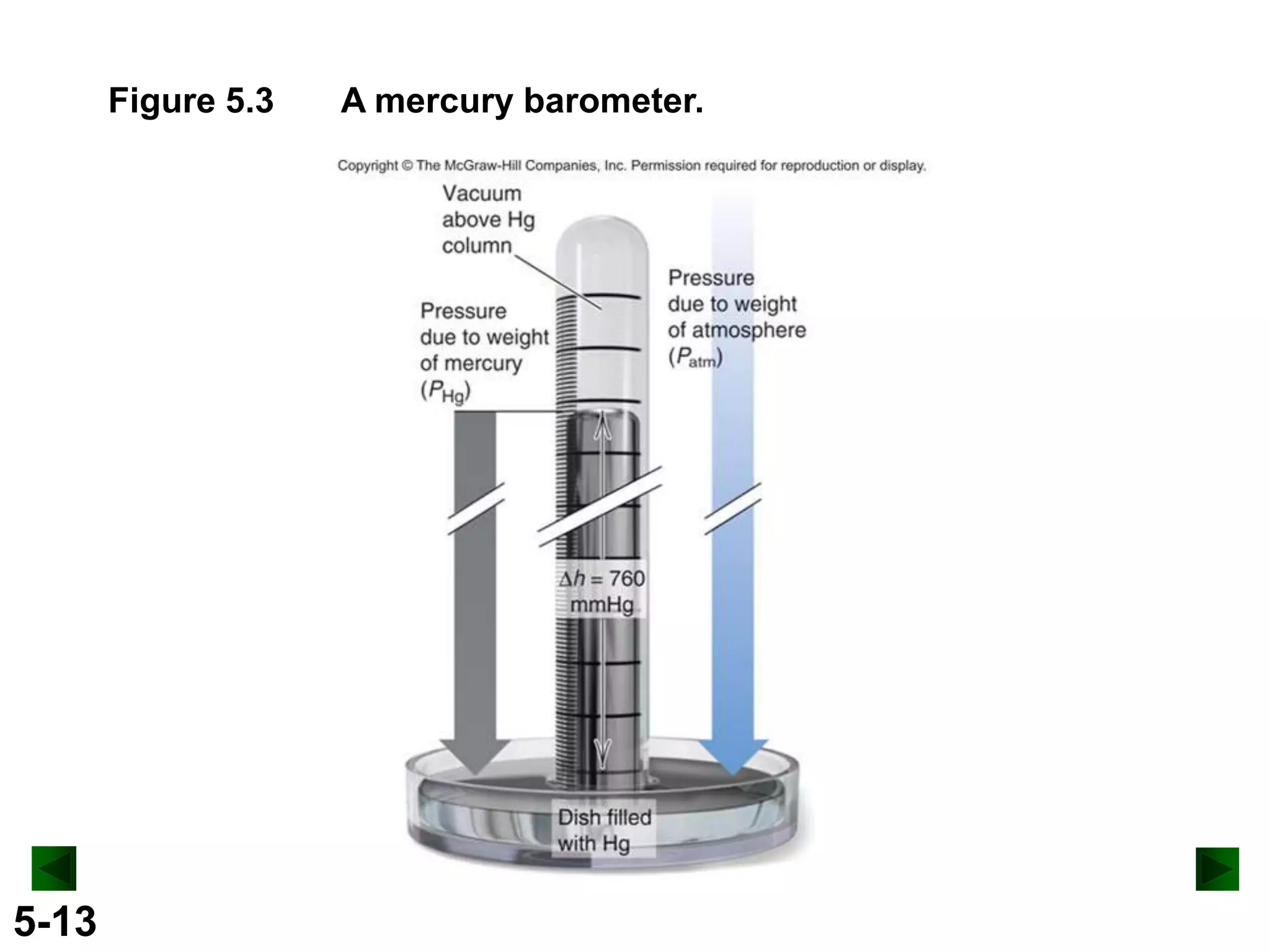
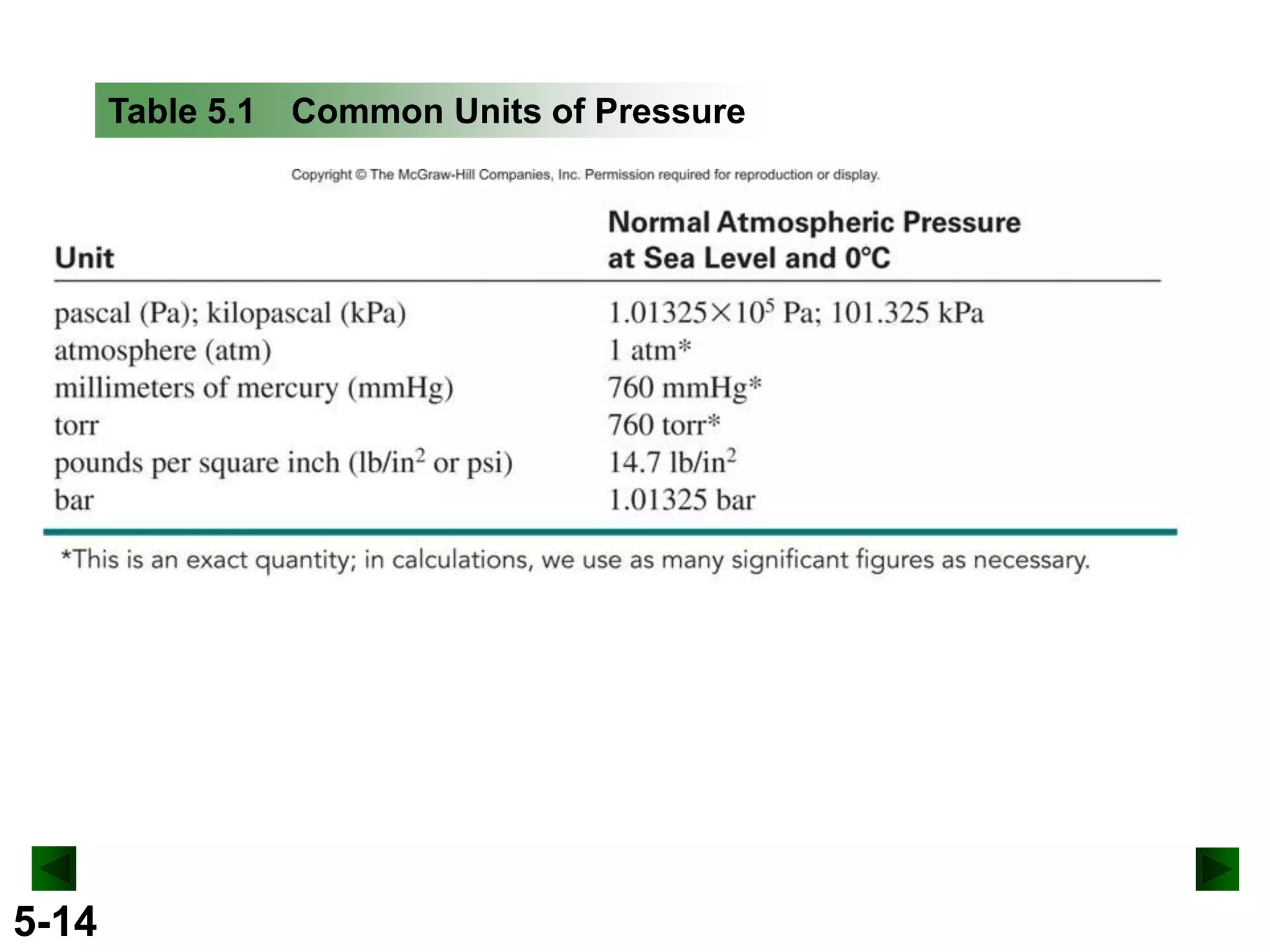
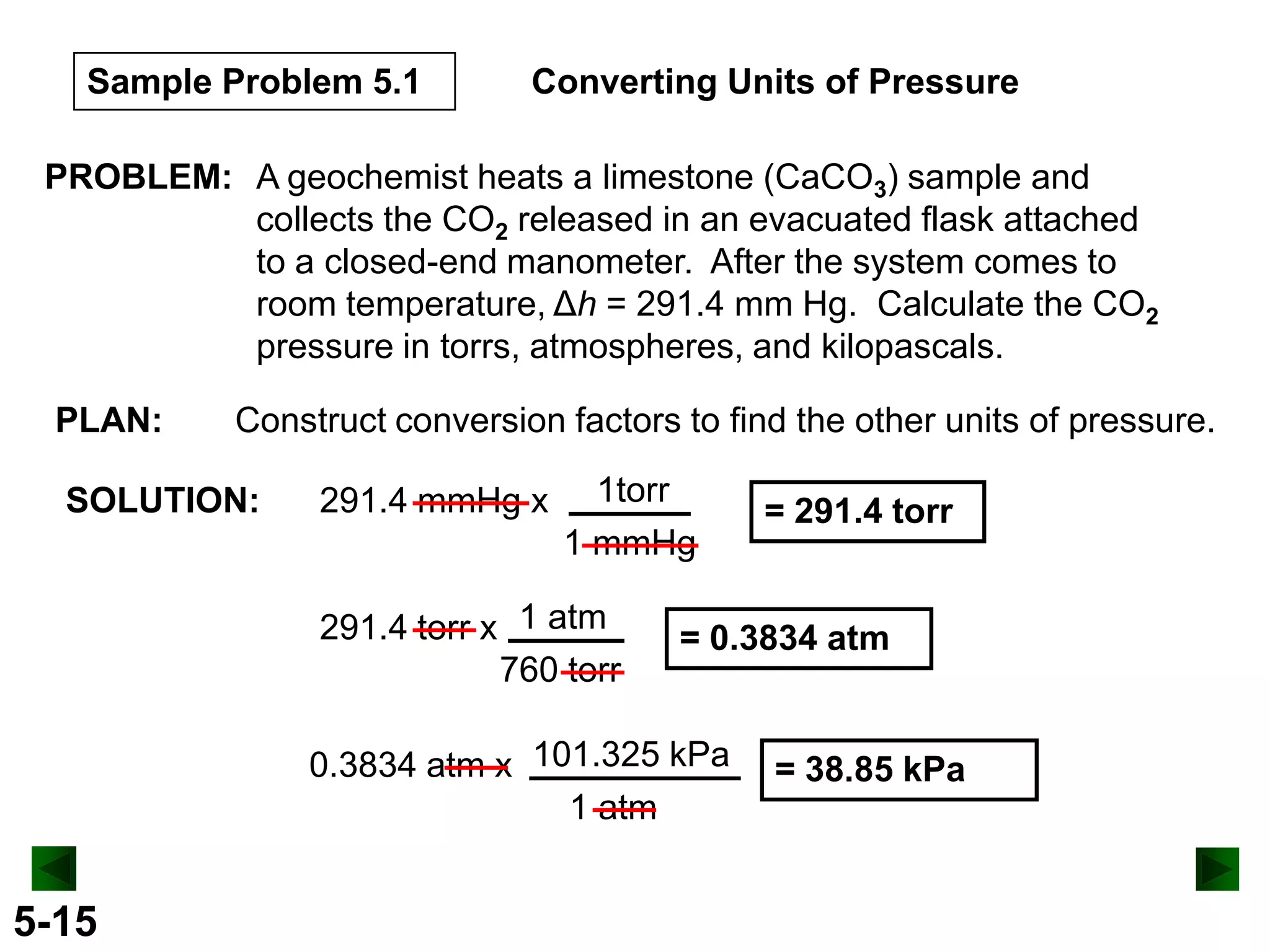
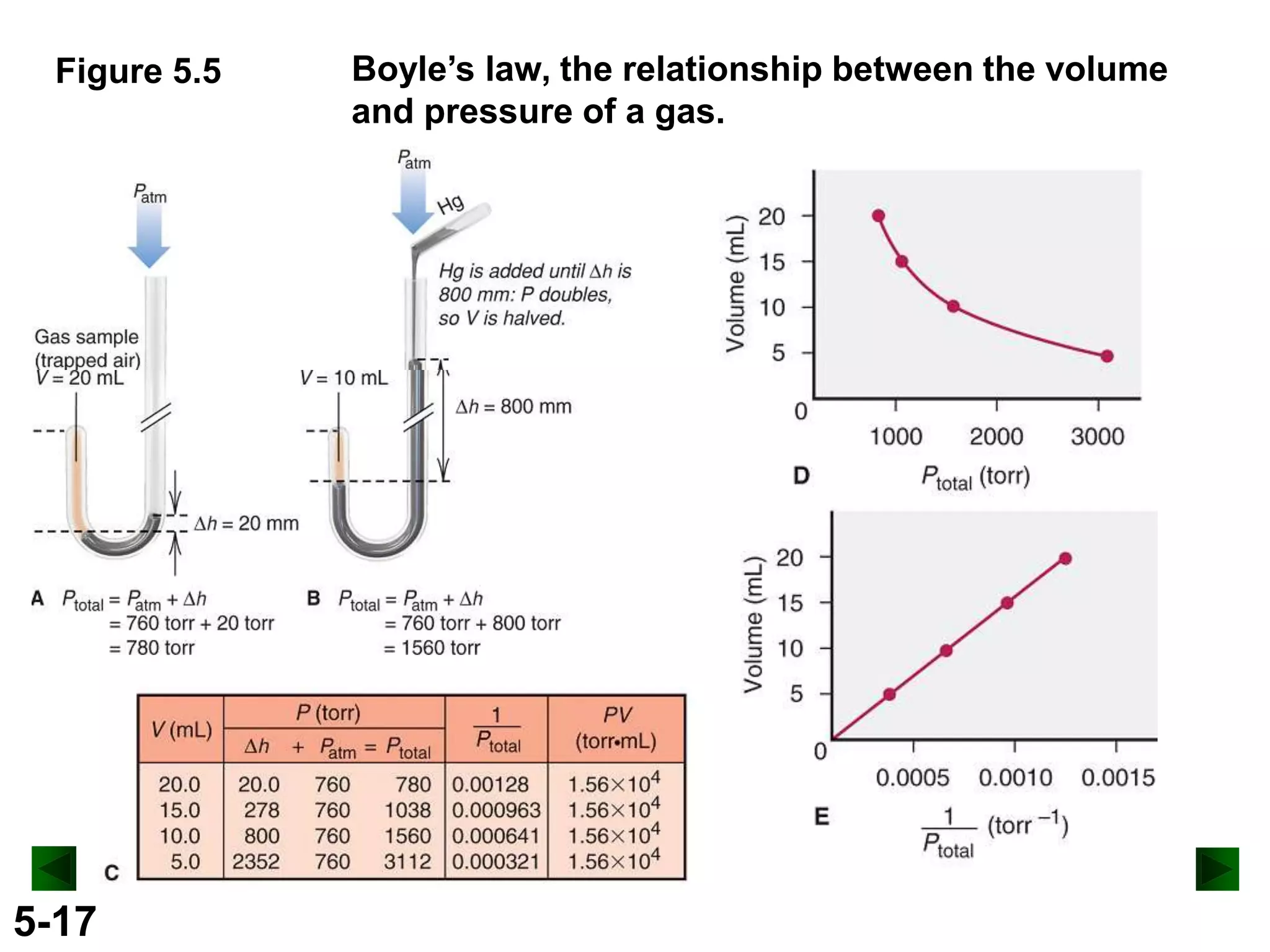
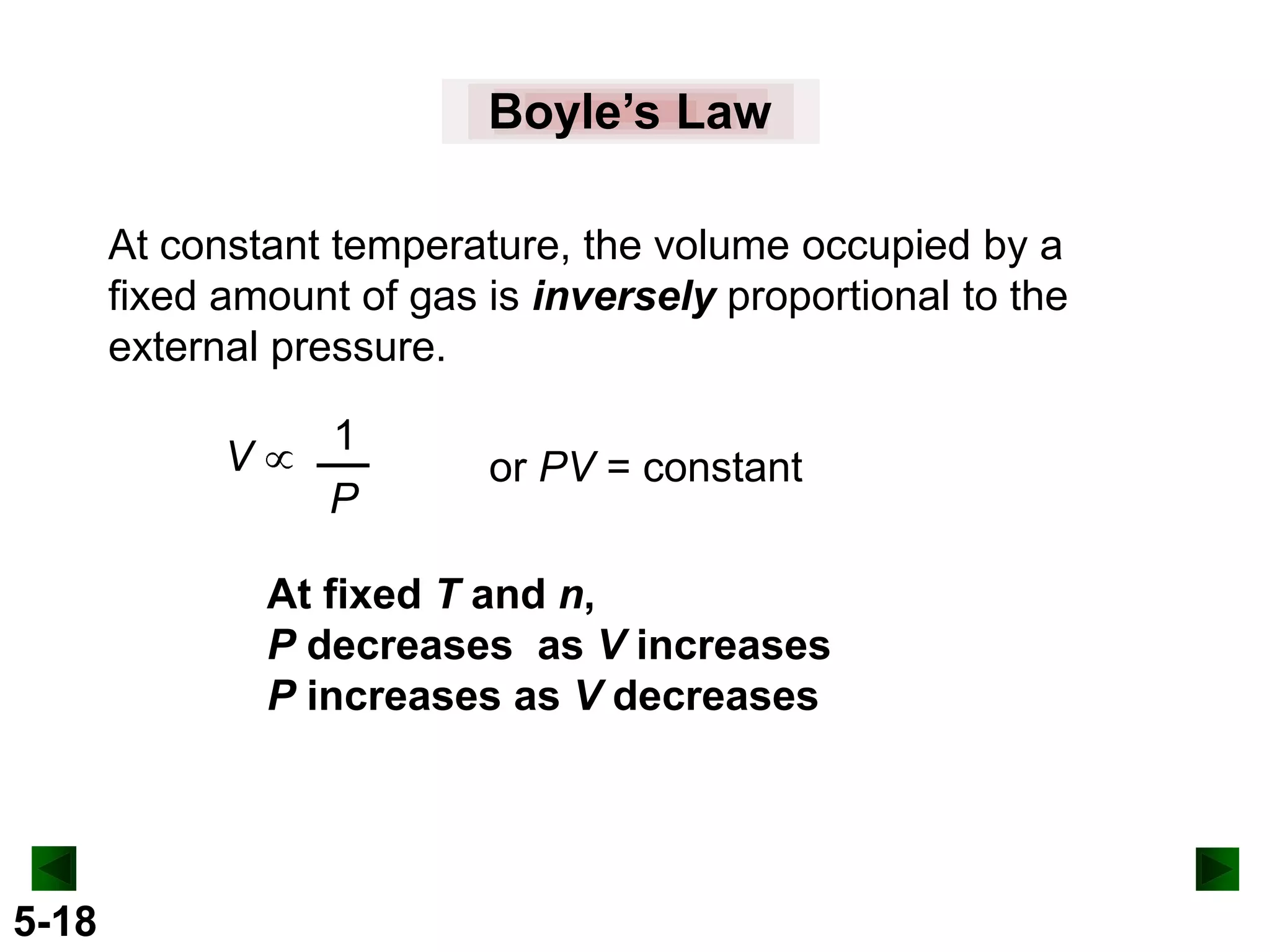
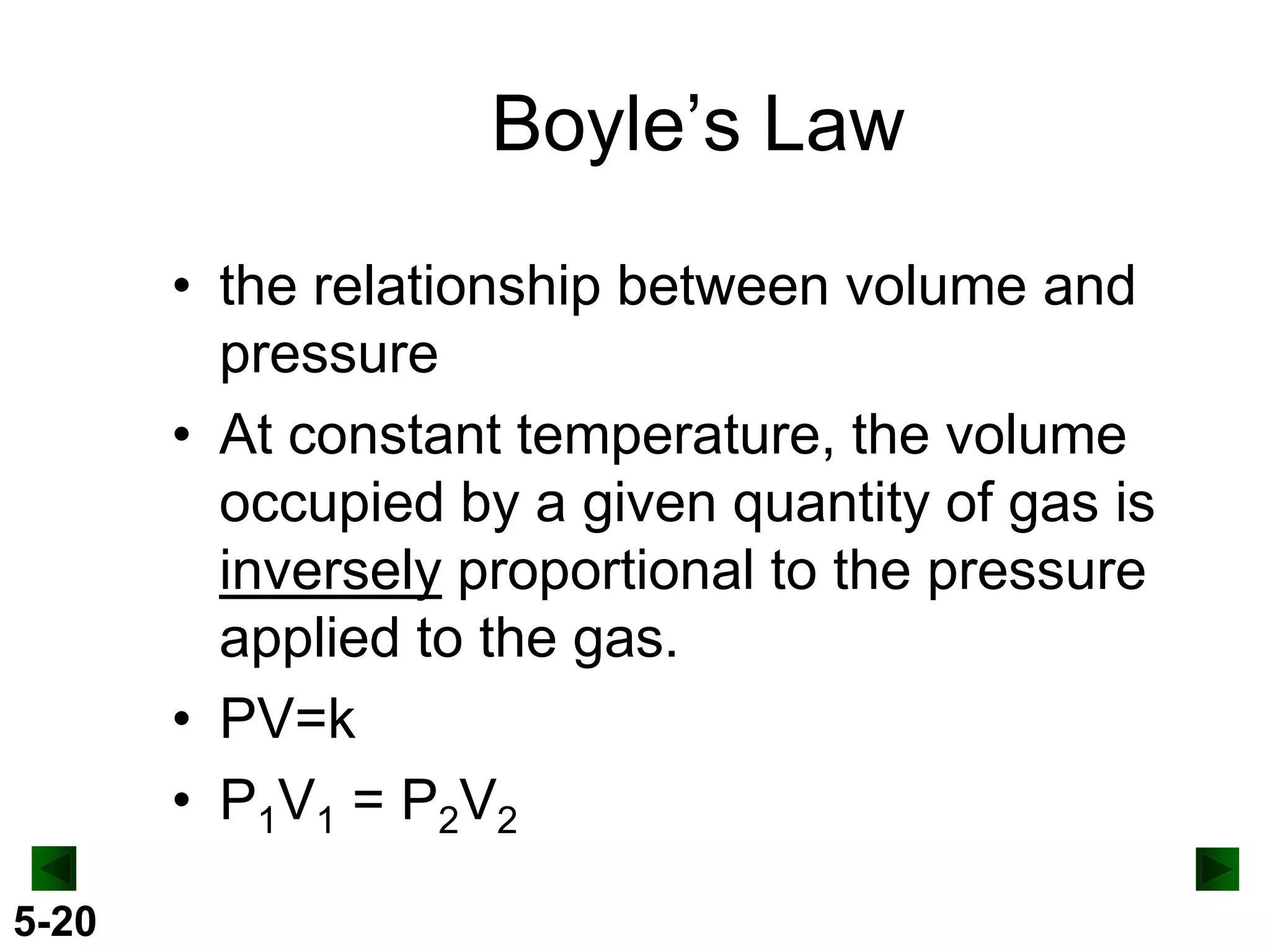
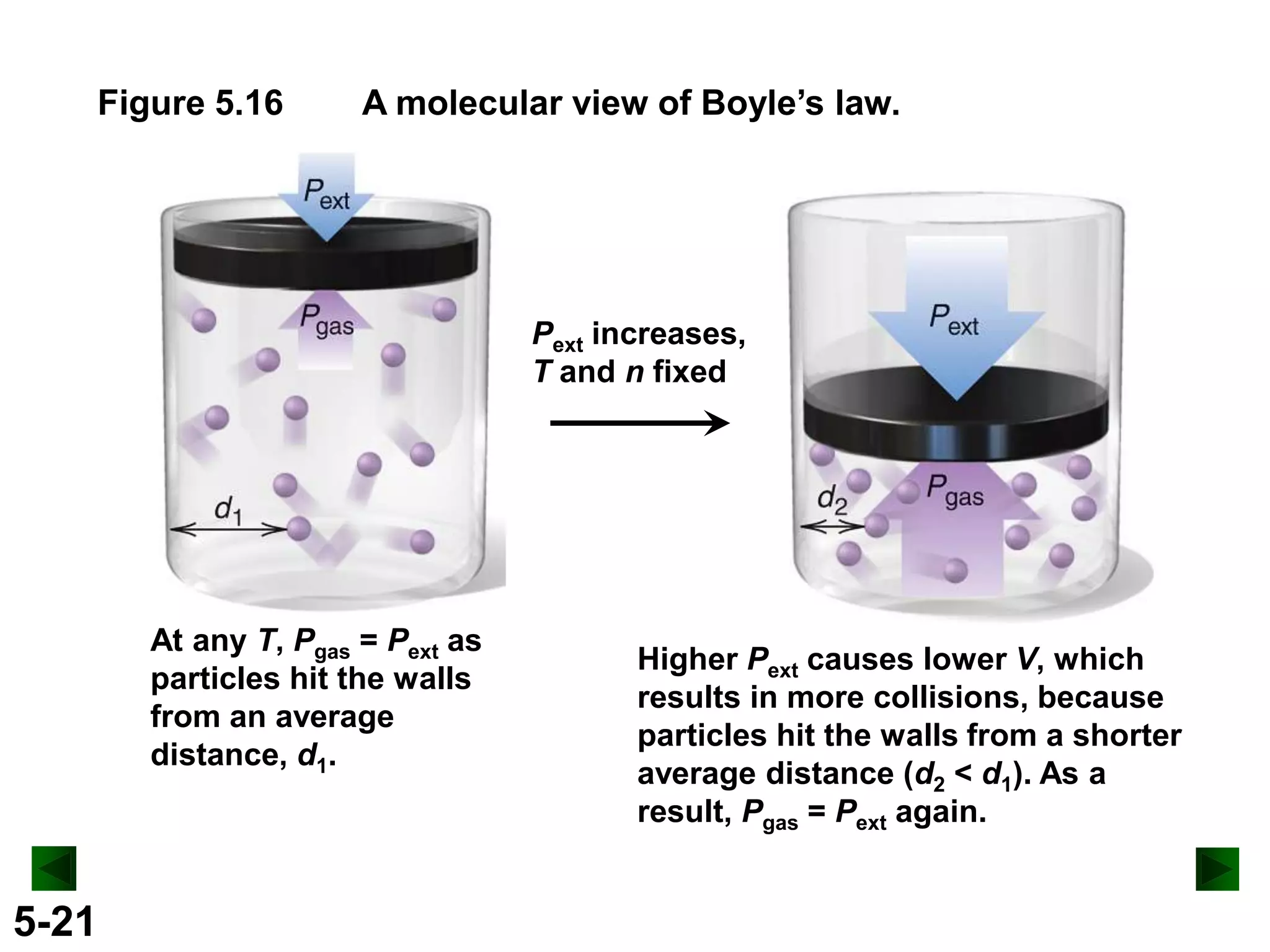
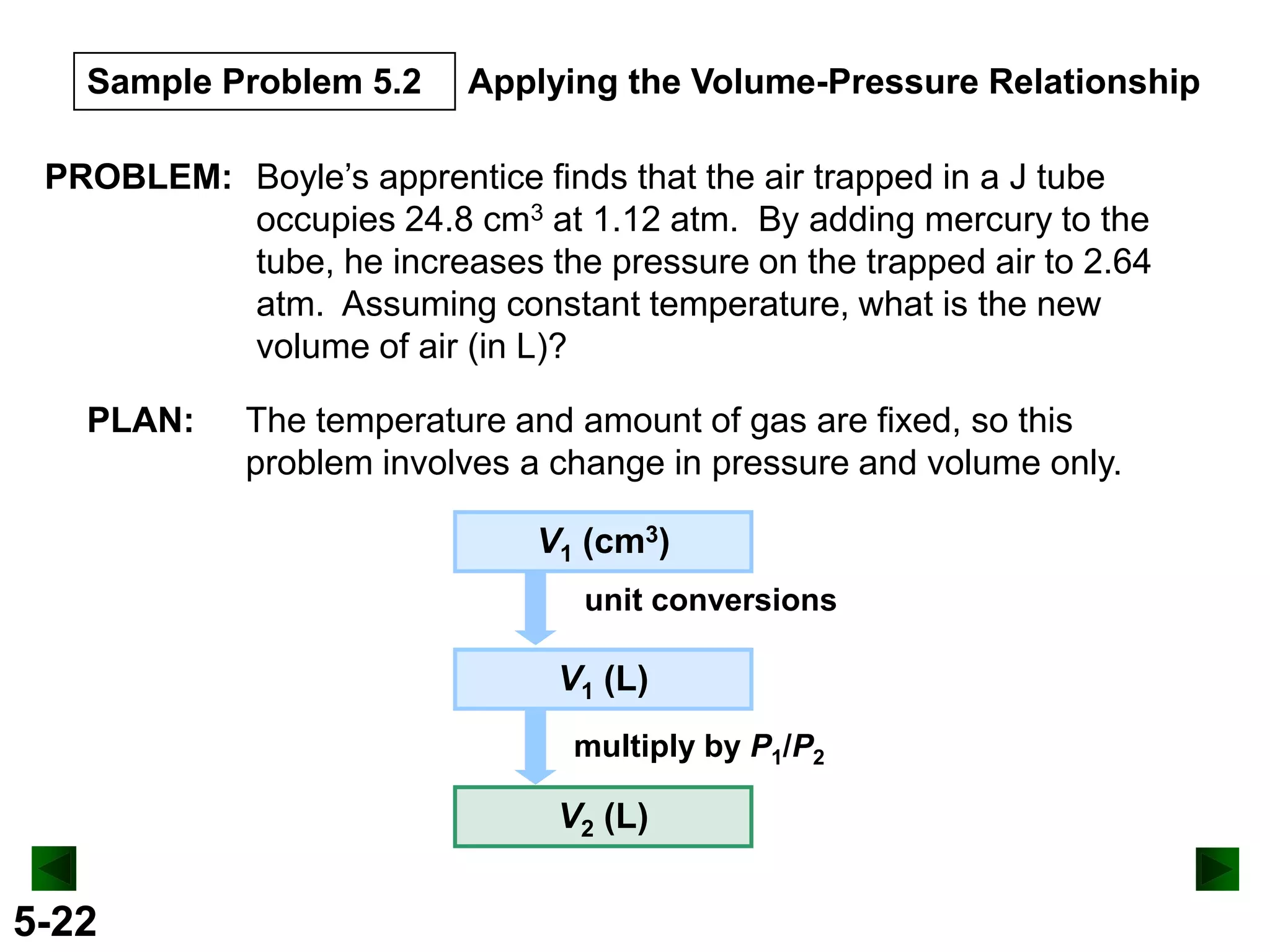
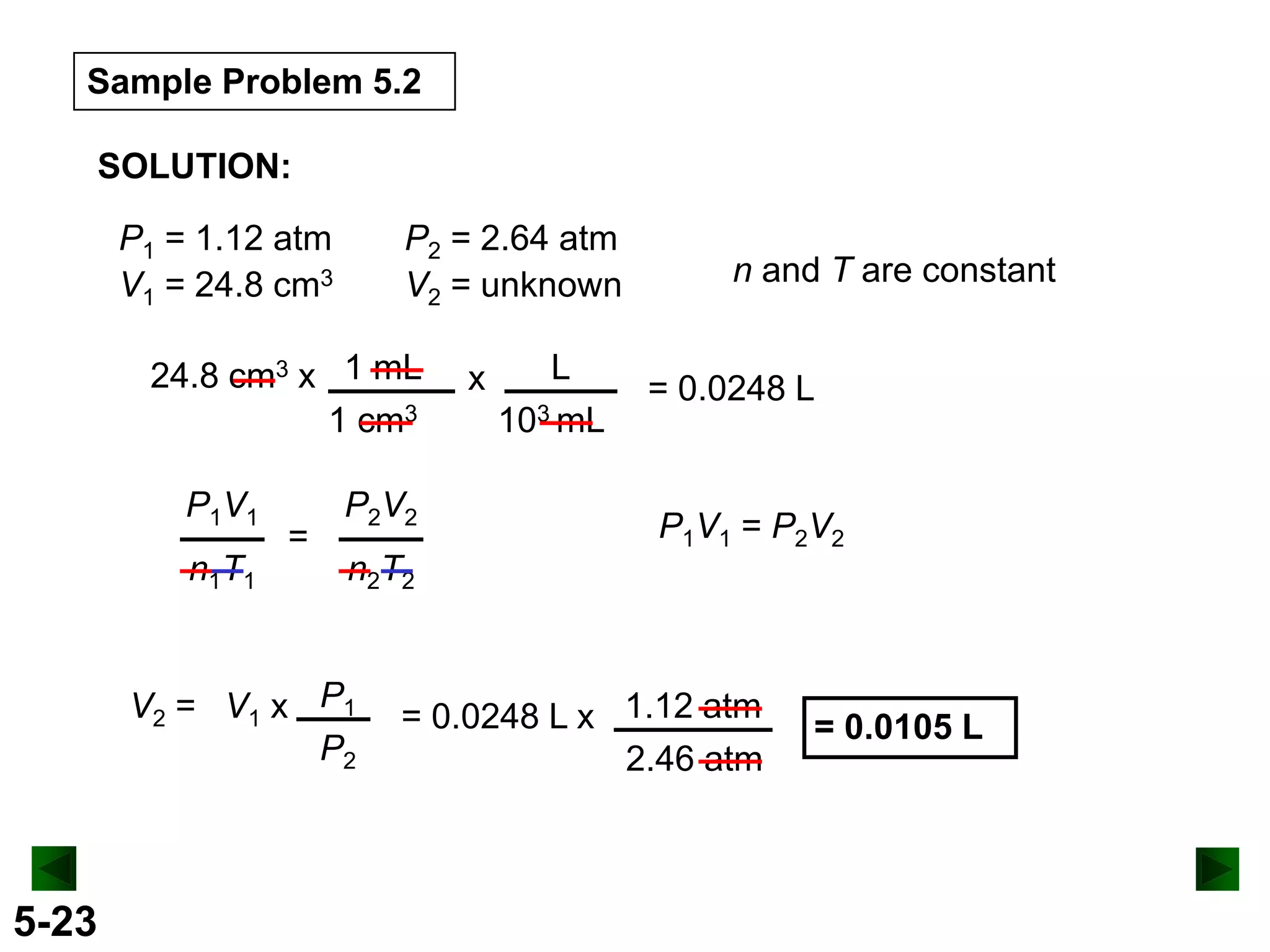
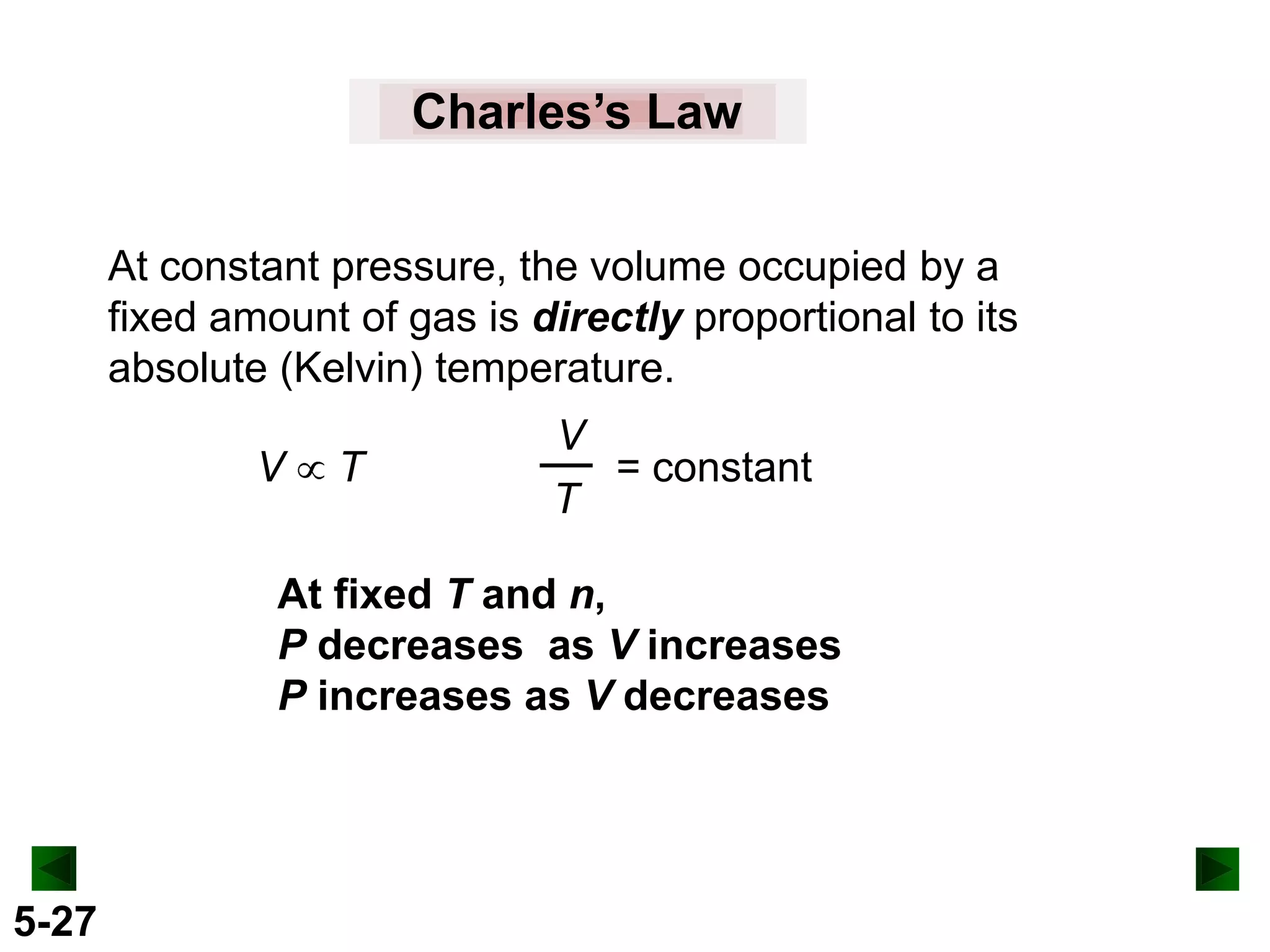
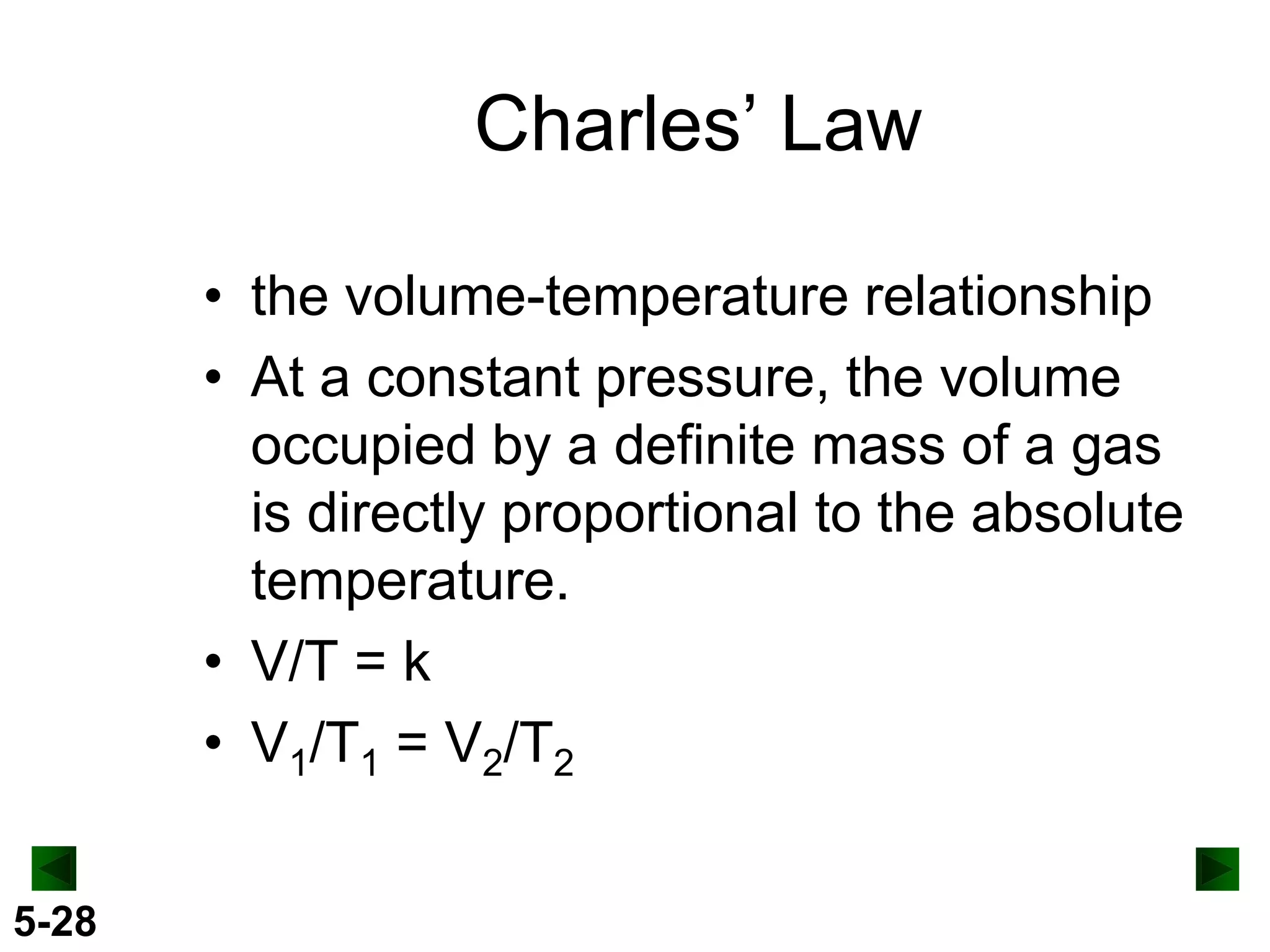
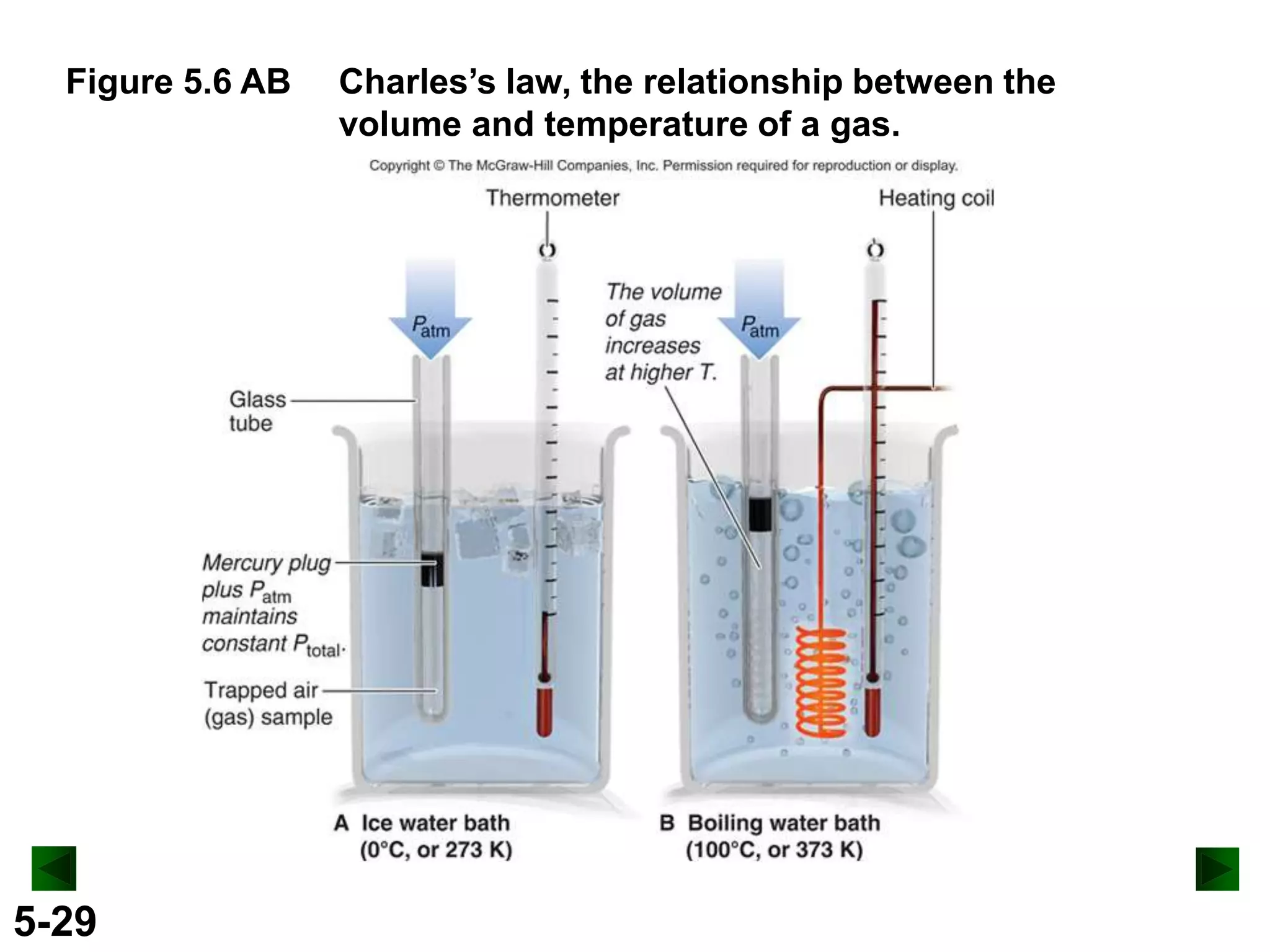
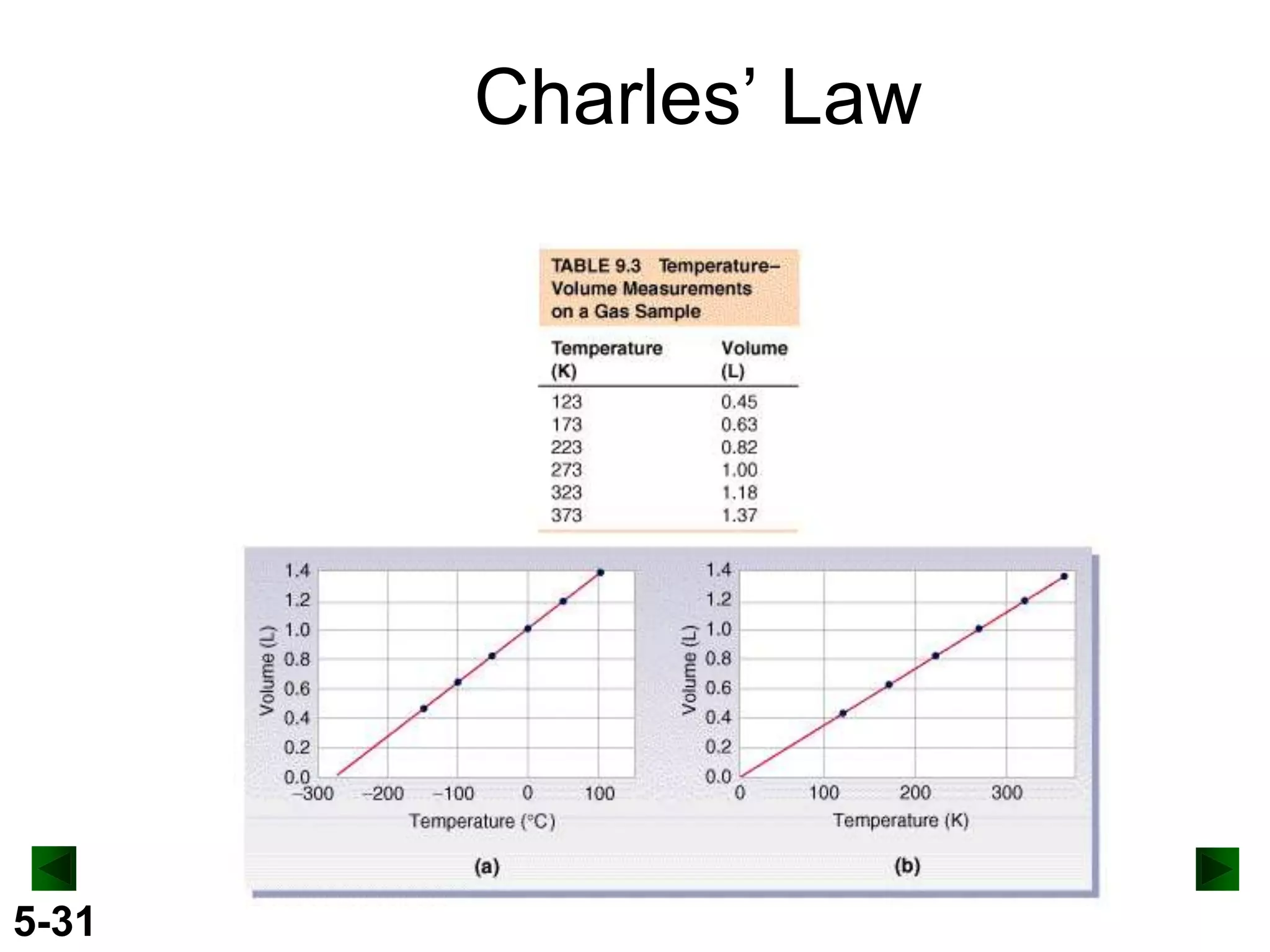
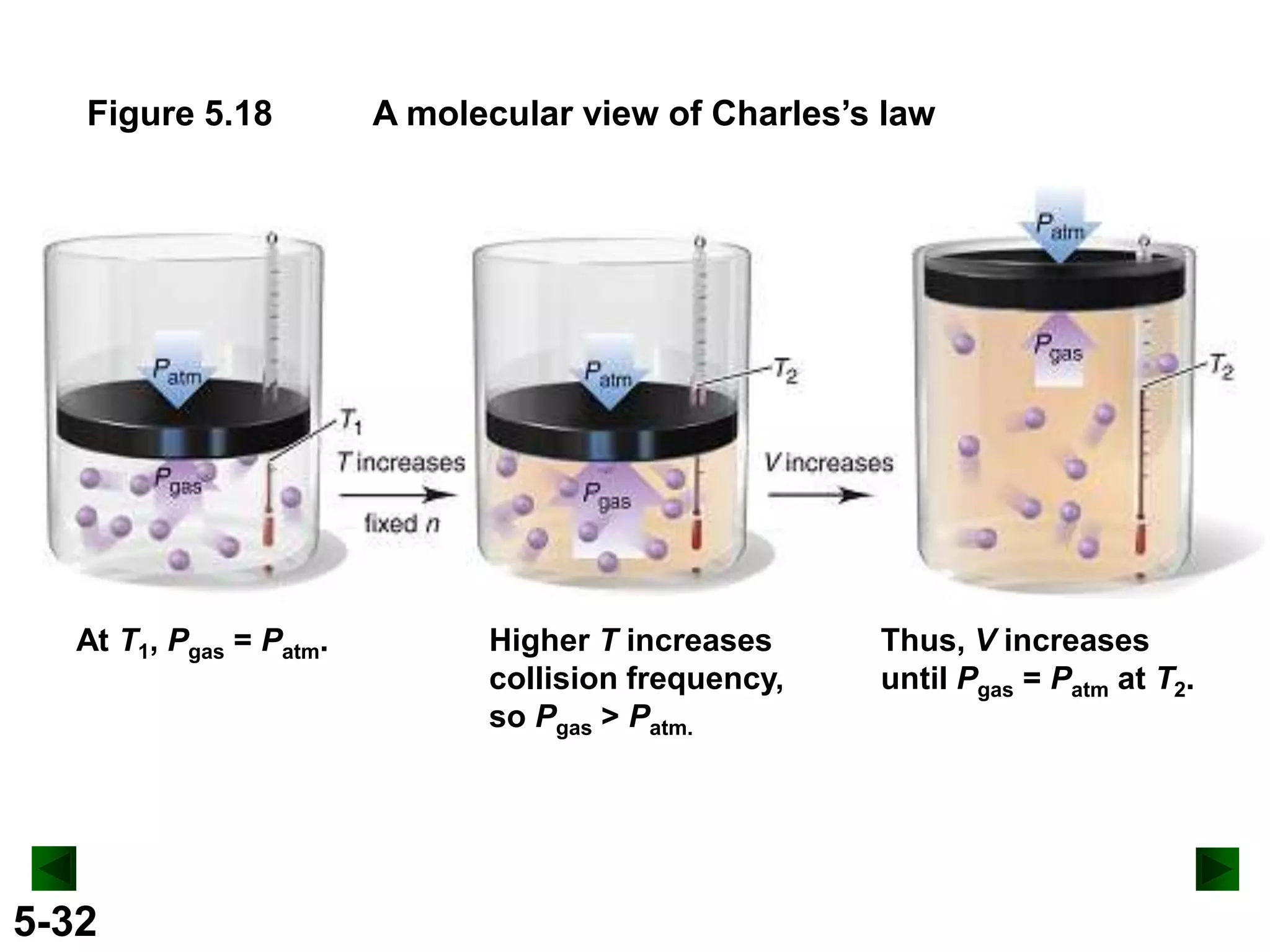
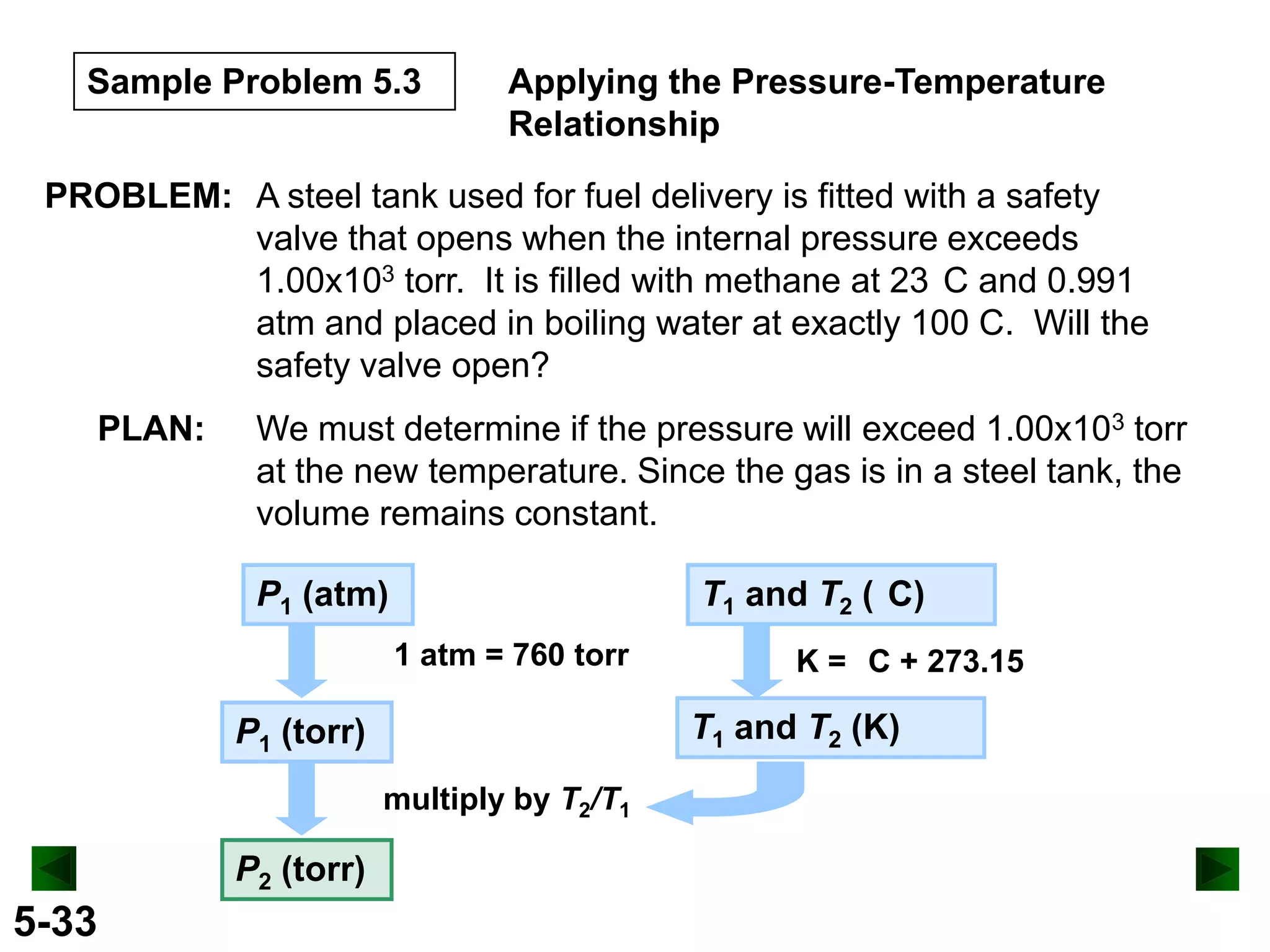
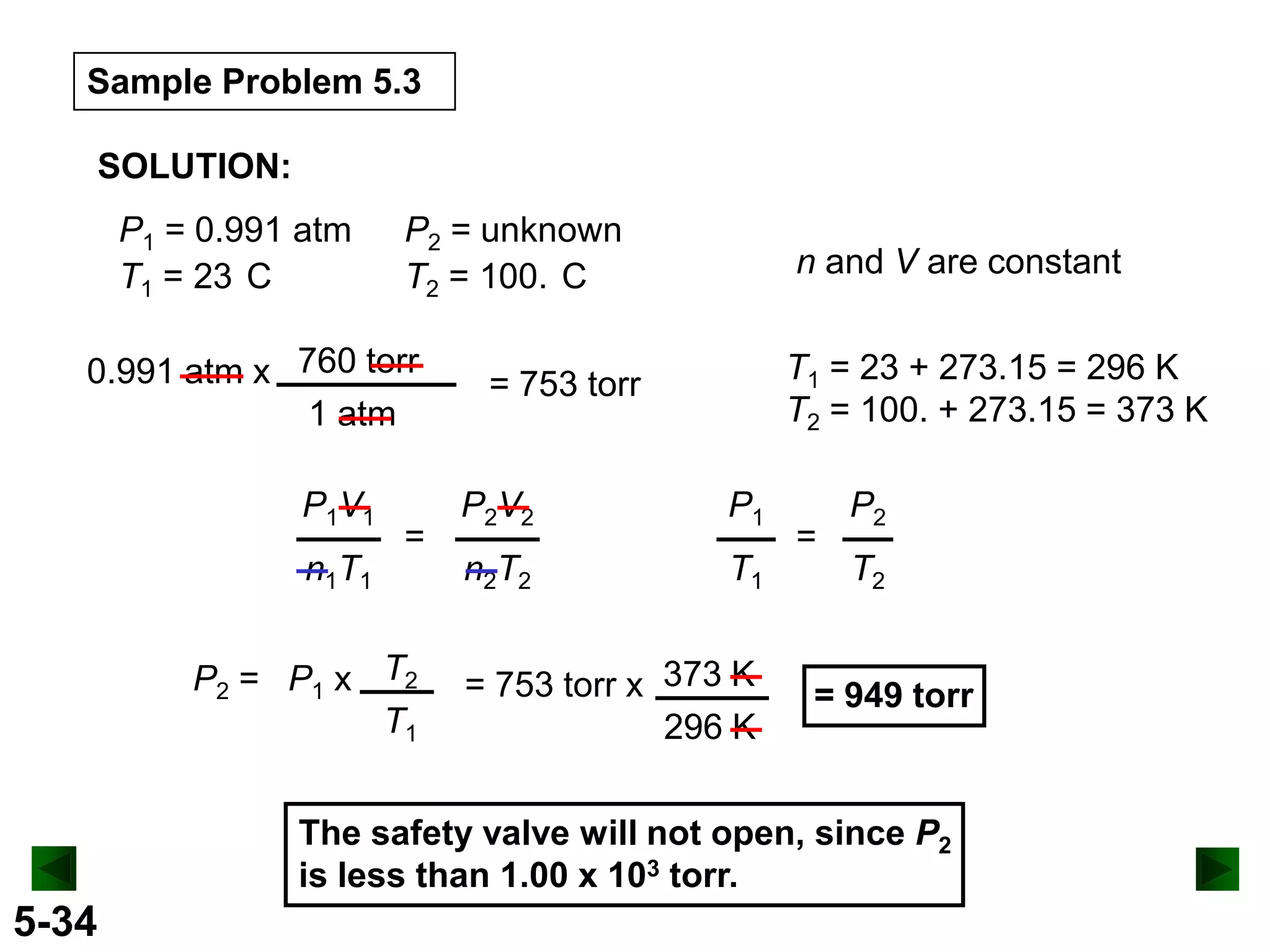
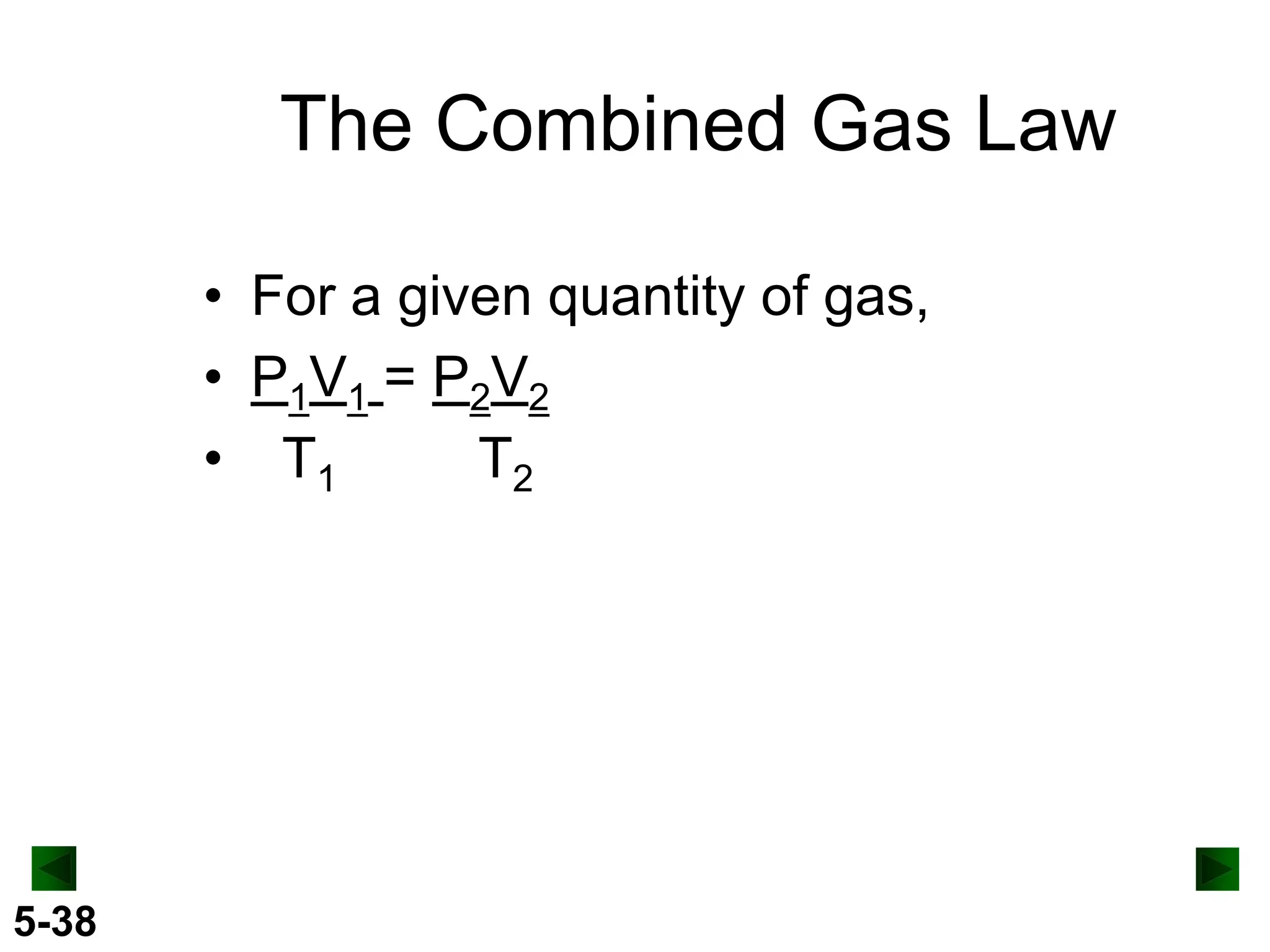
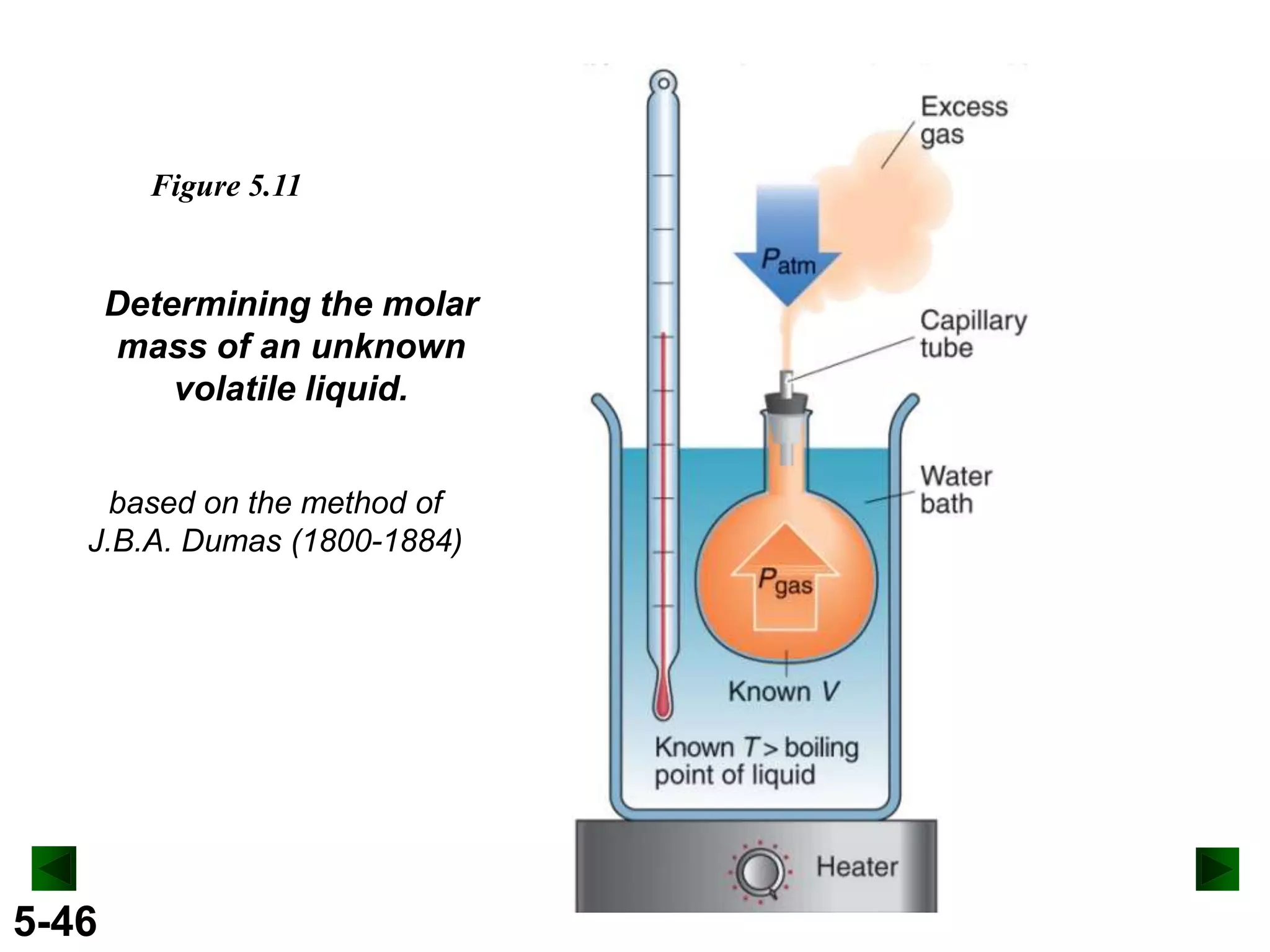
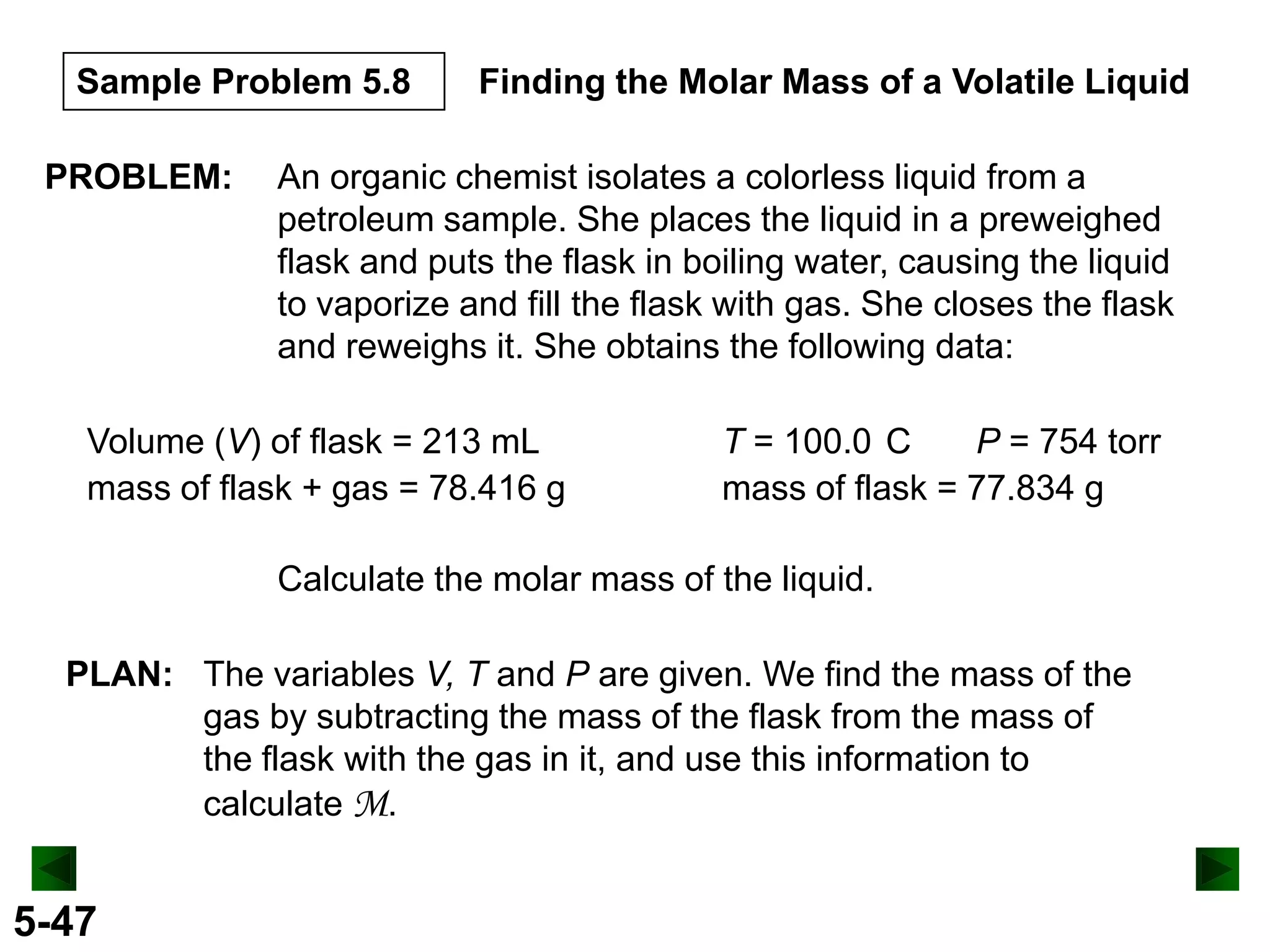
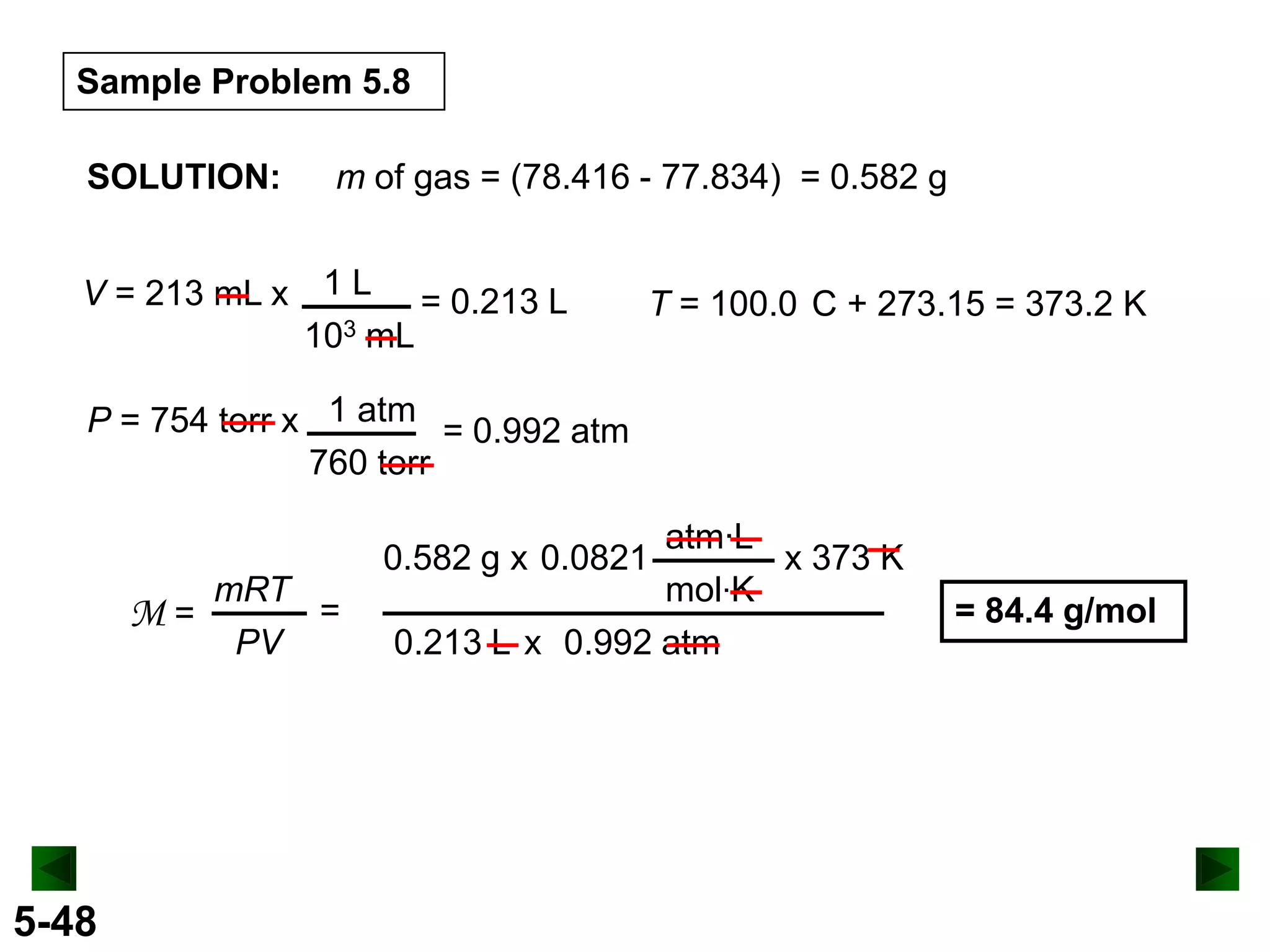
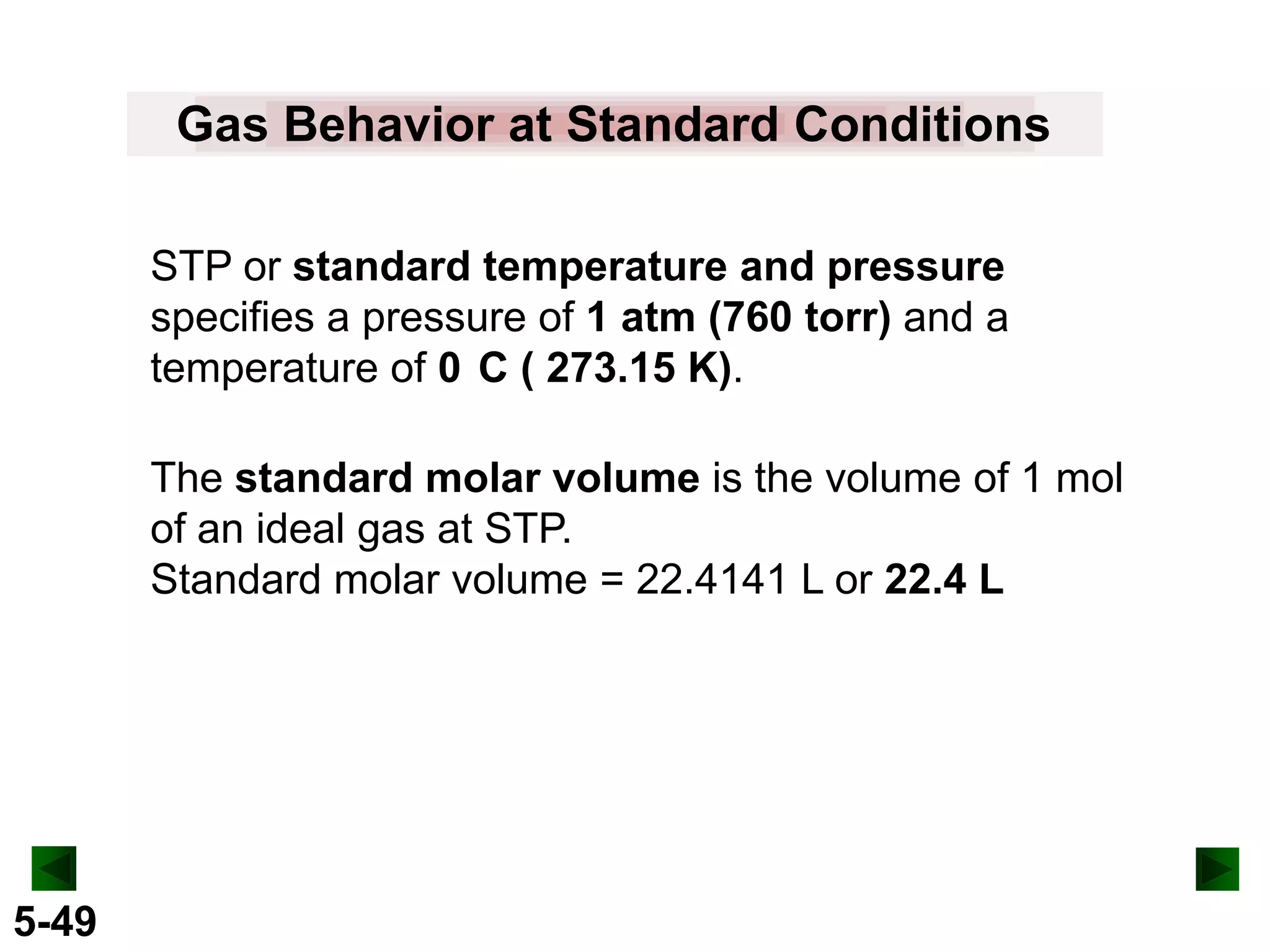
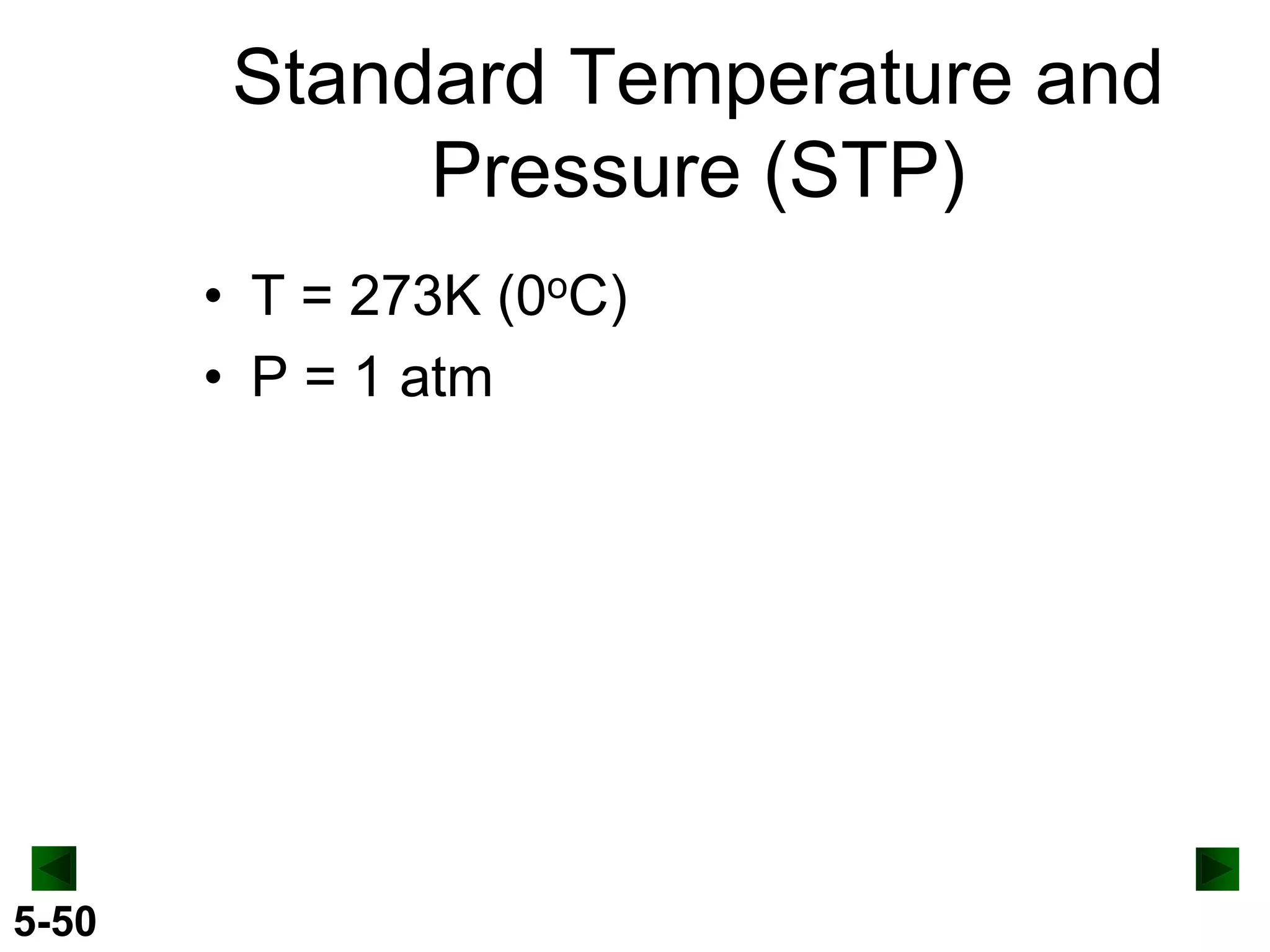
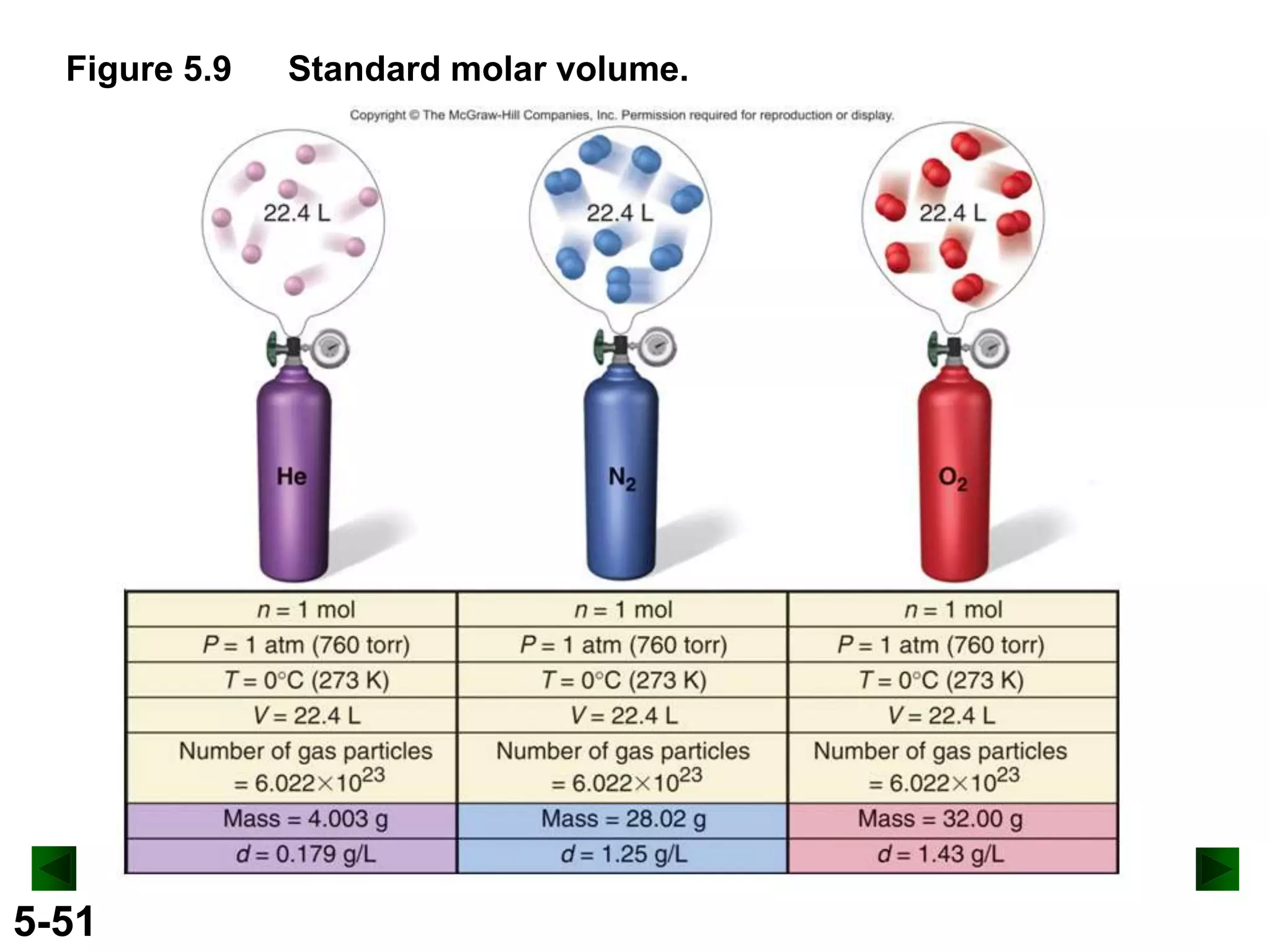
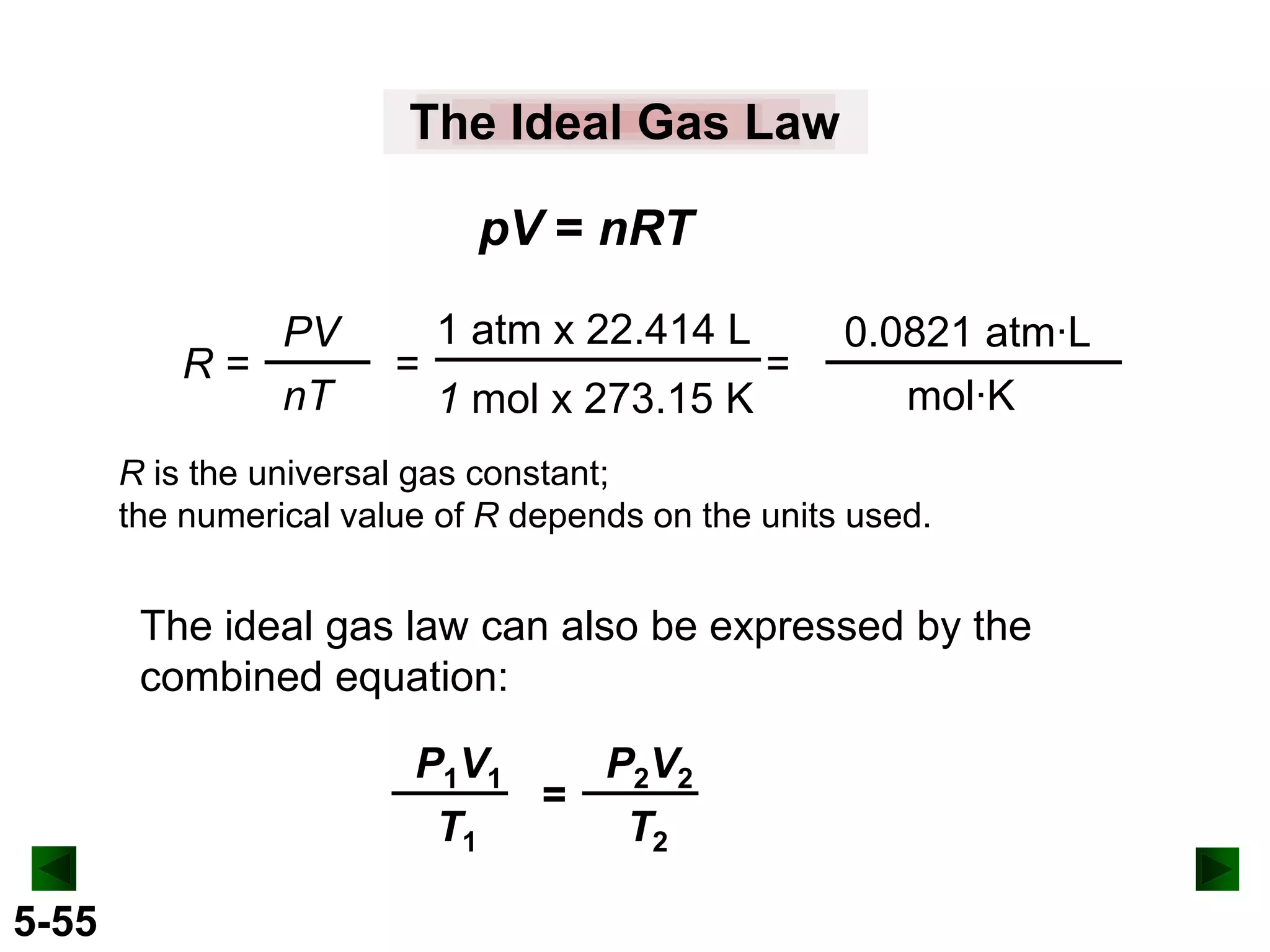
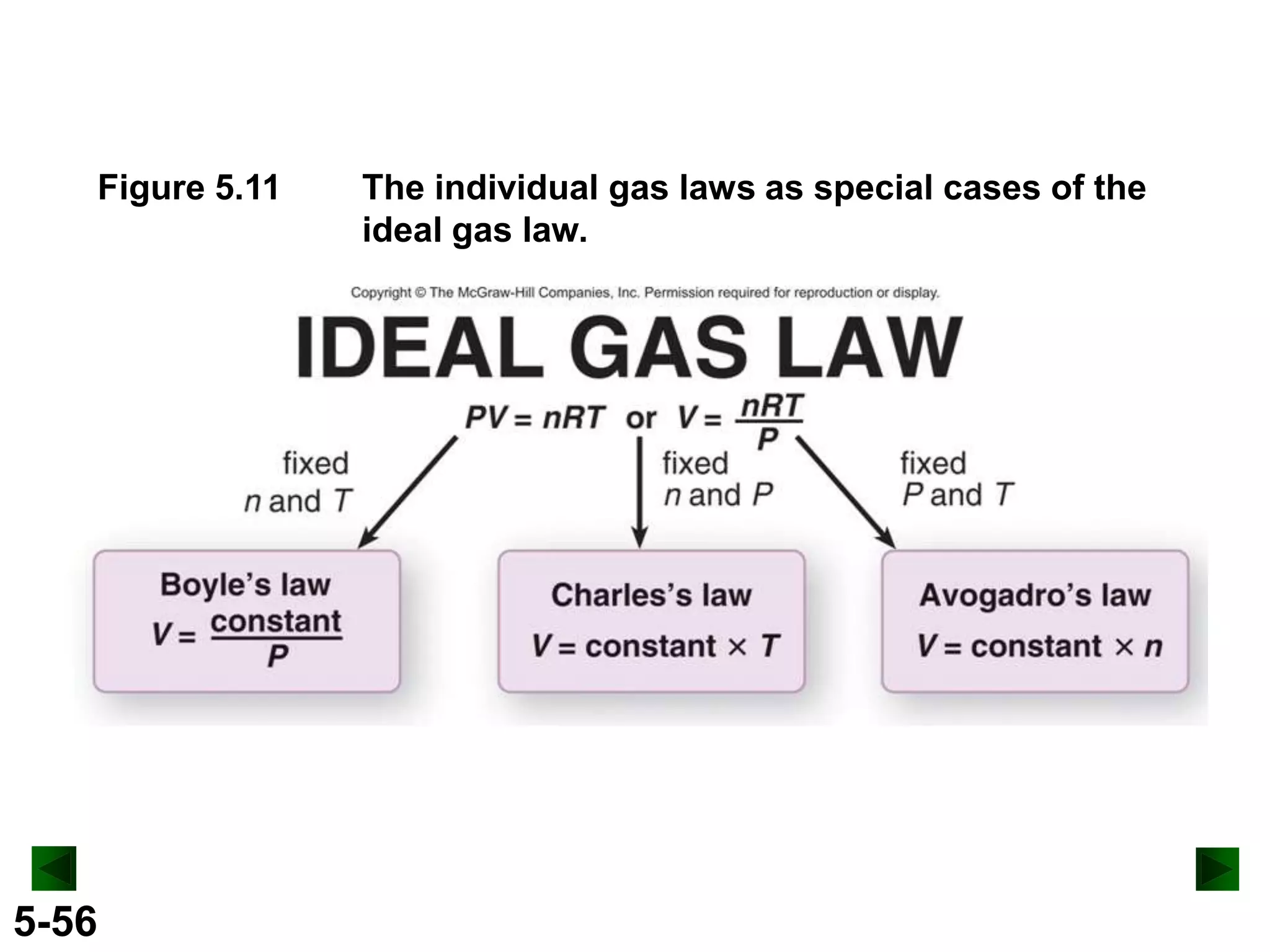
This document provides an overview of gas laws and the behavior of gases. It begins by defining the three states of matter and distinguishing properties of gases. Gas pressure and its measurement are then discussed, including common pressure units. The document outlines the major gas laws - Boyle's Law relating pressure and volume at constant temperature, Charles' Law relating volume and temperature at constant pressure, and the Combined Gas Law combining these relationships. Examples are provided to demonstrate applications of the gas laws. The ideal gas law is defined as relating pressure, volume, temperature, and moles of gas. The behavior of gases at standard temperature and pressure is also covered.