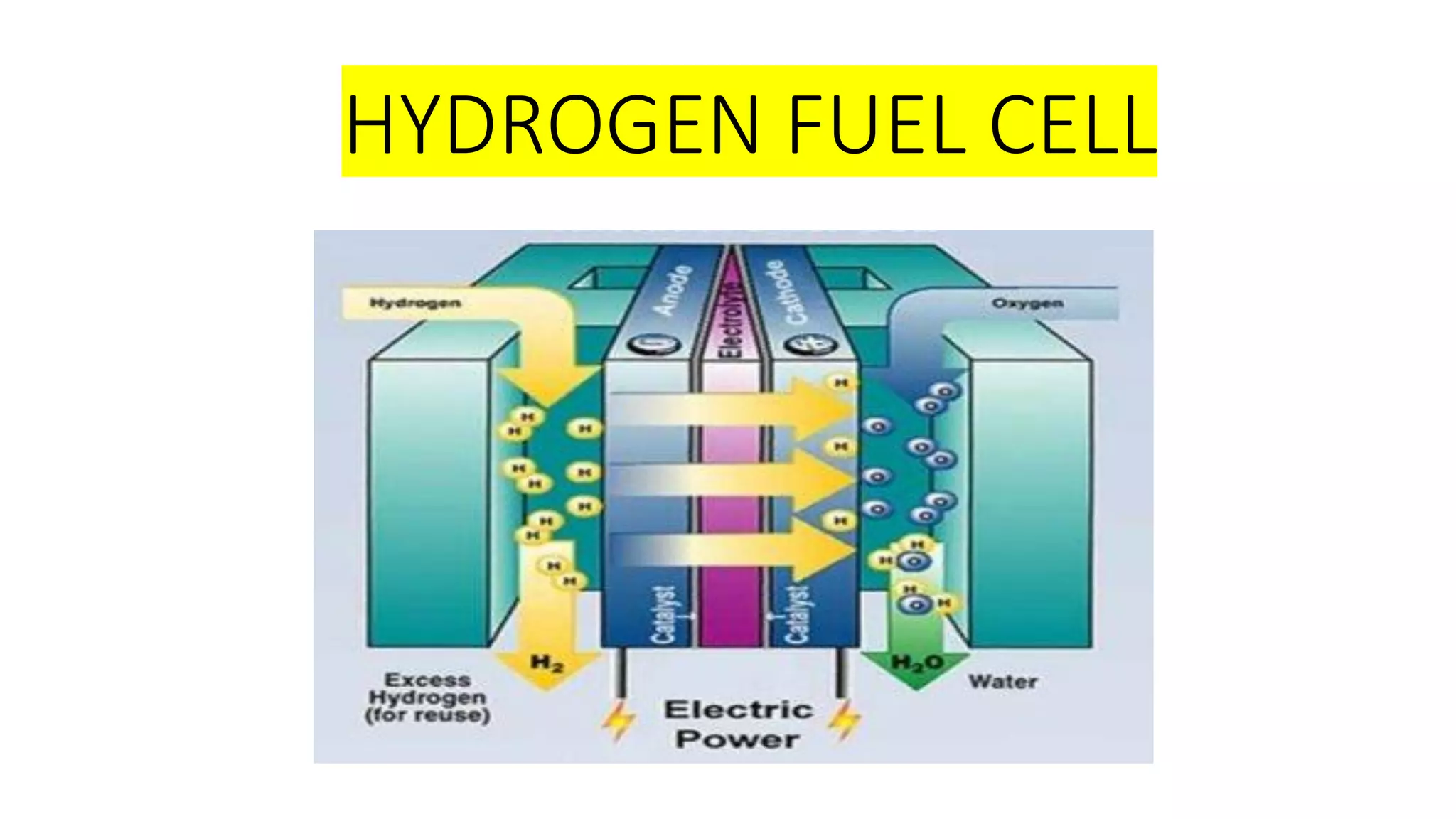
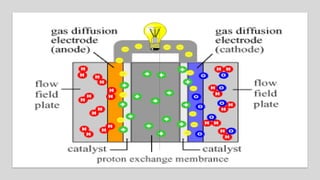
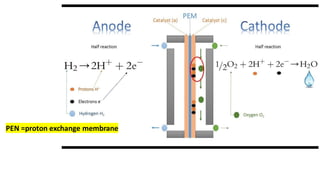
1. Hydrogen fuel cells produce clean energy through an electrochemical reaction between hydrogen and oxygen that results in electricity, heat, and water.
2. Hydrogen is stored and fed to the anode where it is split into protons and electrons, the protons pass through the electrolyte while the electrons power an external circuit.
3. At the cathode, oxygen and the protons react to form water, completing the circuit and reaction.