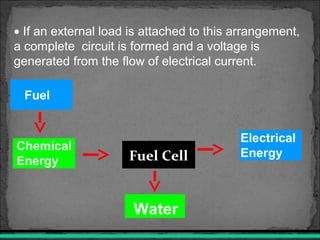
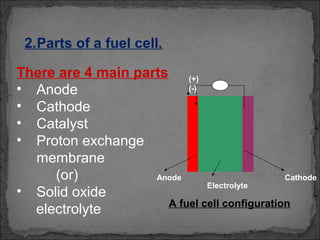
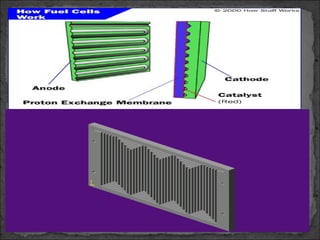
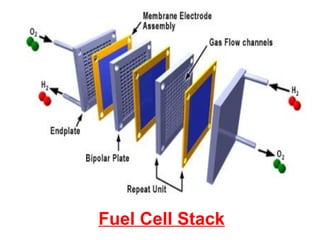
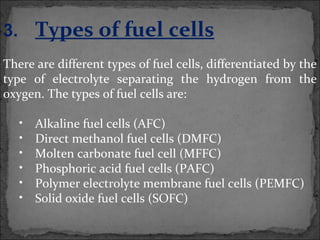
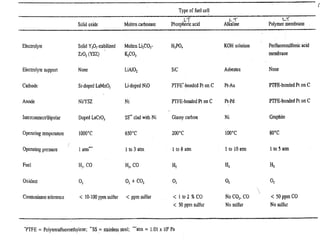
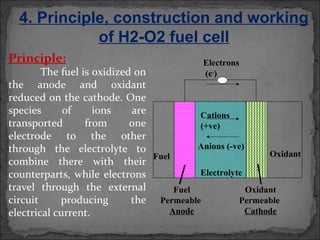
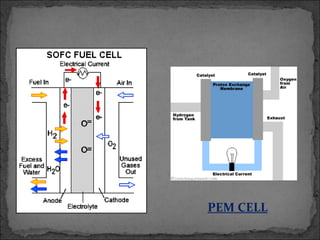
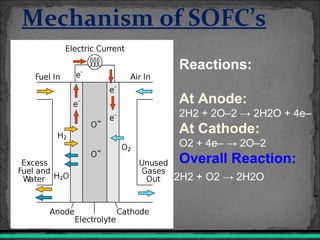
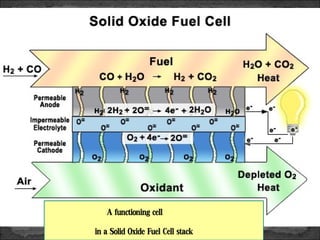
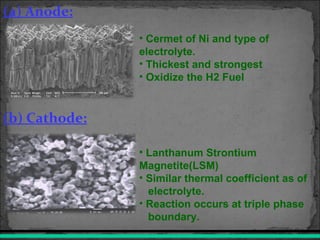
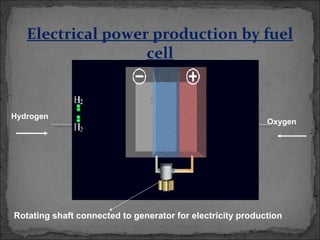
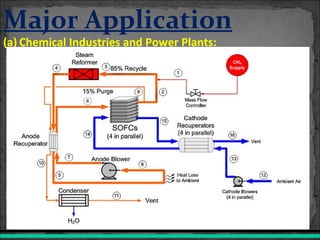
A fuel cell converts hydrogen and oxygen into electricity, heat, and water through an electrochemical reaction. It has four main parts: an anode, cathode, catalyst, and proton exchange membrane. There are different types of fuel cells that use various electrolytes. Fuel cells have advantages like high efficiency, zero emissions, and quiet operation. Applications include stationary power sources, transportation, portable devices, and distributed power generation. Research continues to improve fuel cell performance and reduce costs.