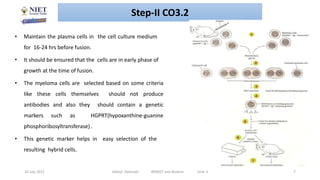
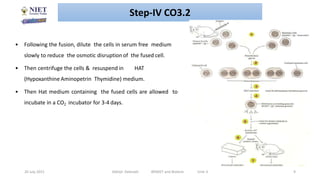
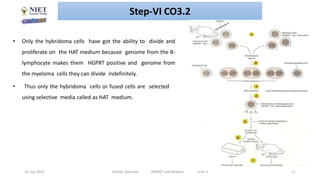
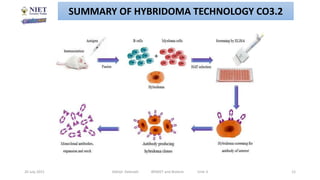
Hybridoma technology involves fusing antibody-producing B lymphocytes with myeloma tumor cells to produce hybridoma cells. These hybridoma cells are immortal and divide indefinitely while continuously producing monoclonal antibodies of a single specificity. The key steps involve immunizing mice, fusing their spleen cells containing B lymphocytes with myeloma cells using polyethylene glycol, and selecting antibody-producing hybridomas by culturing the fused cells in HAT selective medium. This technique allows for the unlimited production of monoclonal antibodies that recognize a single antigen.