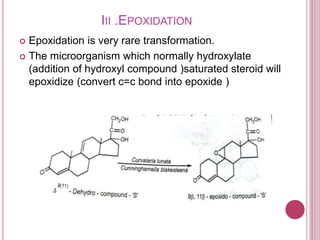
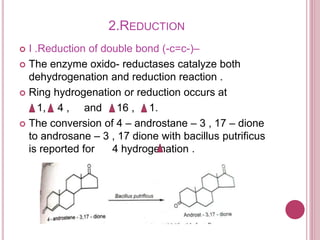
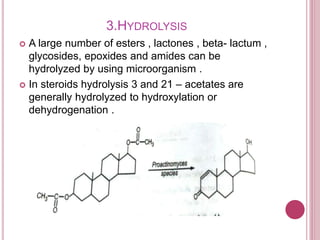
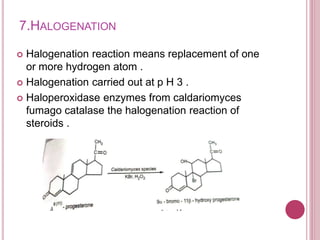
Microbial biotransformation uses microorganisms like bacteria, fungi, and actinomycetes to modify organic compounds through enzymatic reactions. Key reactions include oxidation, reduction, hydrolysis, and others. These transformations are used commercially to produce pharmaceuticals, vitamins, antibiotics, and other chemicals. For example, microbes can hydroxylate steroids through oxidation or reduce ketones and aldehydes. Biotransformation offers advantages like selectivity and mild reaction conditions compared to chemical synthesis.