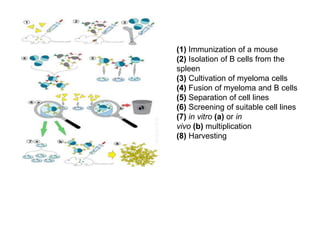
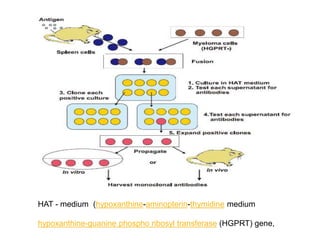
The document discusses monoclonal antibodies (mAbs) and the history of hybridoma technology, first discovered in 1975 to produce mAbs specific to antigens through cell fusion. It outlines the advantages and disadvantages of mAbs, including their cost-effectiveness and challenges in production. Additionally, it details the technical processes involved in hybridoma technology, its applications in medicine, and references for further reading.