The document discusses the structures and properties of quinoline, isoquinoline, and indole. Quinoline and isoquinoline are fused aromatic ring systems consisting of benzene fused to pyridine with the nitrogen at different positions. Indole is a fused aromatic ring system consisting of benzene fused to pyrrole. The structures are described including hybridization and delocalized pi orbitals. Common preparation methods are outlined such as Skraup synthesis for quinoline and Bischler-Napieralski synthesis for isoquinoline. Key chemical reactions including electrophilic substitution, reduction, and reactions with acids and bases are also summarized.



![Preparations
1. Skraup Synthesis:
Here a mixture of glycerol (propane- 1,2,3-triol), aniline
(phenylamine), sulfuric acid, nitrobenzene and ferrous [iron(II)]
sulfate are heated together.
The reaction is exothermic and tends to become very violent.
ferrous [iron(II)] sulfate is added to make the reaction less
violent.
Nitrobenzene, or an alternative oxidant (iodine or chloroaniline
are often recommended), is required to convert the product, 1,2-
dihydroquinoline into quinoline.
Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP 4](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-4-2048.jpg)







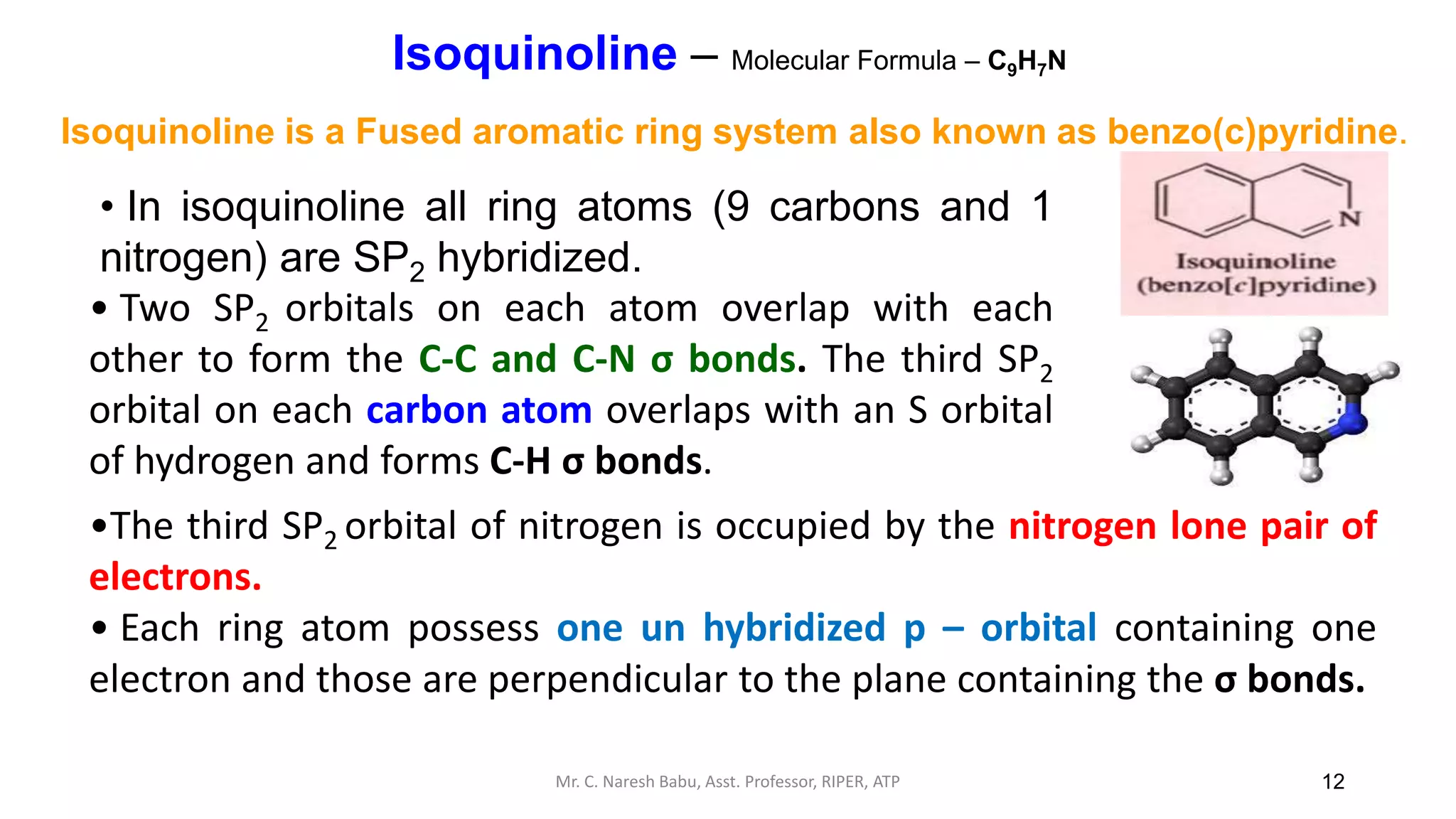
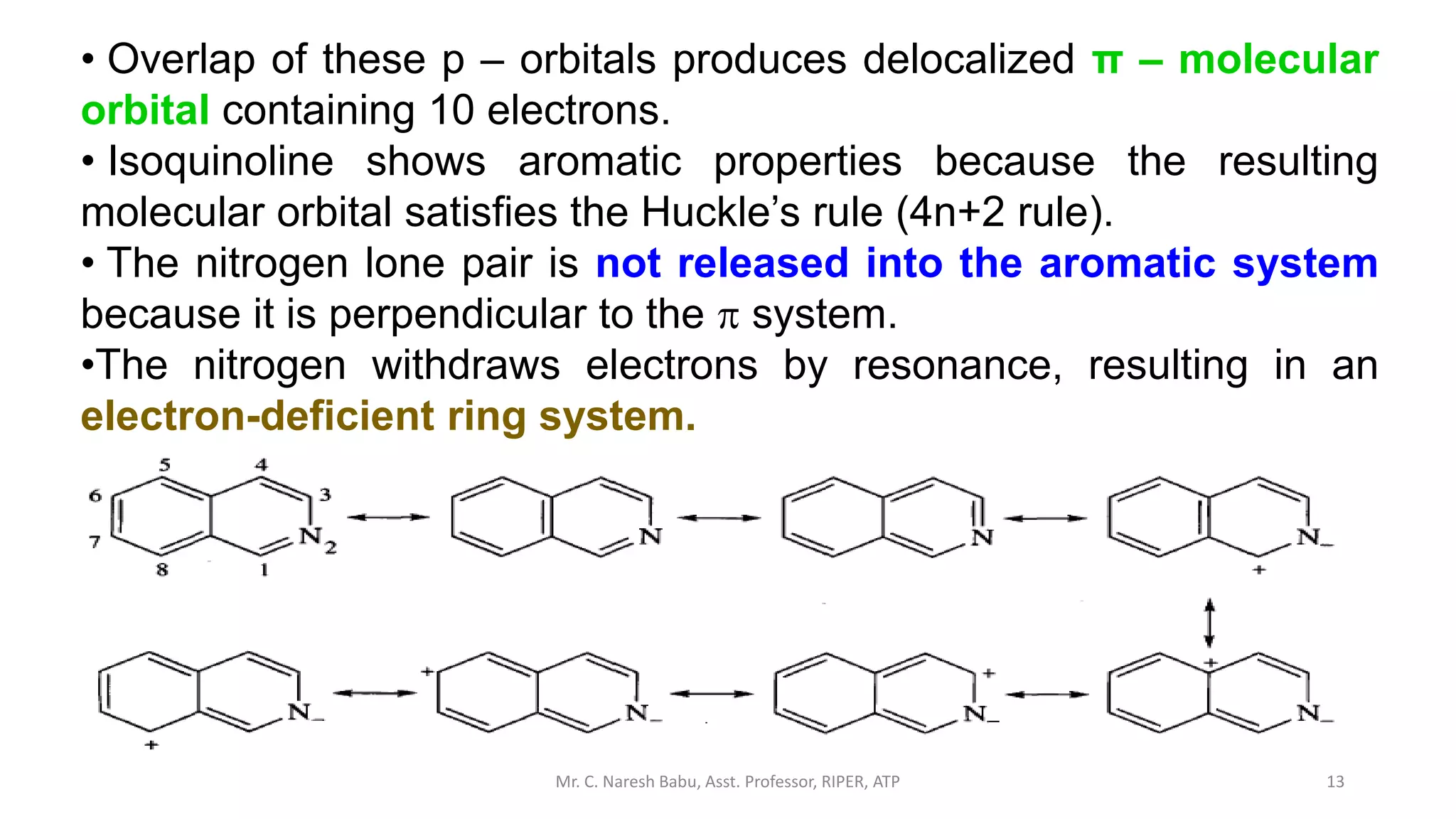




















![34
Acridine – Molecular Formula – C13H9N
• In acridine all ring atoms (13 carbons and 1
nitrogen) are SP2 hybridized.
• Two SP2 orbitals on each atom overlap with each
other to form the C-C and C-N σ bonds. The third SP2
orbital on each carbon atom overlaps with an S orbital
of hydrogen and forms C-H σ bonds.
Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP
Acridine is a Fused aromatic ring system also known as Dibenzo[b,e]pyridine
/ 2,3-Benzoquinoline
•The third SP2 orbital of nitrogen is occupied by the lone pair of electron
of nitrogen.
• Each ring atom in ring possess one un hybridized p – orbital containing
one electron and those are perpendicular to the plane containing the σ
bonds.](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-34-2048.jpg)









![Basic Character & Acidic character:
• Benzimidazole is a weak base and also a weak acid.
• Benzimidazole is a weak base because due to the presence of
lone pair of electrons on one of the nitrogen atom.
• Benzimidazole also exhibit weak acidic properties, the weak acidic
property is because of its formation of potassium phenothiazine
with KOH.
Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP 44
N
N
H
Benzimidazole
H Cl
N
N
H
H
Cl
Benzim idazole hydrochloride
N
N
H
K O H
- H 2O
N
N
K1H -benzo[d]im idazole
N -potassium benzimidazole](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-44-2048.jpg)
![Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP 45
Chemical reactions:
Electrophilic Substitution reactions:
Electrophiles attack in benzimidazole take place preferentially at the 5- or 6-
position. However, the electrophile may also enter the 4- or 7-position if the 5- or 6-
position is blocked.
Nitration:
Sulphonation:
N
N
H
B e n z i m i d a z o l e
H N O 3
N
N
H
O 2 N
5 - n i t r o - 1 H - b e n z o [ d ] i m i d a z o l e
N
N
H
B e n z i m i d a z o l e
H 2 S O 4
N
N
H
H O 3 S
1 H - b e n z o [ d ] i m i d a z o l e - 5 - s u l f o n i c a c i d](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-45-2048.jpg)

![Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP 47
Halogenation: When 2,5 (or 2,6)-dimethylbenzimidazole in an aqueous acid solution is
treated with a saturated solution of bleaching powder at 0 to 5°C, 1-chloro-2,5(or 2,6)-
dimethylbenzimidazole is obtained.
Nucleophilic substitution reaction:
Reaction with sodamide:
N
N
H
B e n z i m i d a z o l e
N a N H 2
N
N
H
N H 2
1 H - b e n z o [ d ] i m i d a z o l - 2 - a m i n e](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-47-2048.jpg)
![Reduction: Mild reduction by Zn & HCl.
But catalyst Ni / Pt reduces both rings and forms octa hydro indole.
Mr. C. Naresh Babu, Asst. Professor, RIPER, ATP 48
N
H
N
N
H
H
N
Z n / H C l
1 H - b e n z o [ d ] i m i d a z o l e 2 ,3 - d i h y d r o - 1 H - b e n z o [ d ] i m i d a z o l e
N
H
N
N
H
H
N
N i / P t
1 H - b e n z o [ d ] i m i d a z o l e o c t a h y d r o - 1 H - b e n z o [ d ] i m i d a z o l e](https://image.slidesharecdn.com/heterocyclicchemistry-fusedringsystems-170222084928/75/Heterocyclic-chemistry-Fused-ring-systems-48-2048.jpg)





