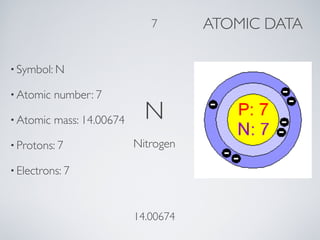
Nitrogen is the most abundant gas in Earth's atmosphere, comprising 78%. Daniel Rutherford discovered nitrogen in 1772 through an experiment where he trapped air in an inverted bottle with a burning candle. The gas that remained after the candle burned out was nitrogen. Nitrogen has many important uses including in fertilizer production, rocket fuels, filling tires, and manufacturing steel and electronics.