heterocycle.ppt
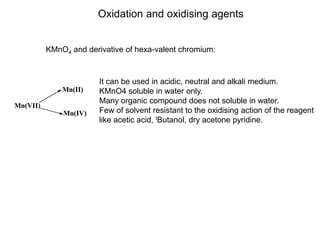
- 1. Oxidation and oxidising agents KMnO4 and derivative of hexa-valent chromium: It can be used in acidic, neutral and alkali medium. KMnO4 soluble in water only. Many organic compound does not soluble in water. Few of solvent resistant to the oxidising action of the reagent like acetic acid, tButanol, dry acetone pyridine. Mn(VII) Mn(II) Mn(IV)
- 2. CH3 KMnO4, rt 18-crown-6 COOH 78% KMnO4, PhH, 25o C dicyclohexano-18-crown-6 O COOH 90% KMnO4, H2O, NaOH,0o C PhCH2N+ Me3Cl- OH OH Using of crown ether is very helpful for oxidation with permanganate. Tetra nbutylammonium permanganate is soluble in organic solvents.
- 3. Cr (VI) to Cr(III), common oxidising agents are CrO3 and sodium or pottassium dichromate. CrO3 is a polymer, exist like (CrO3)n, when dissolve, depolymerisation takes place. (CrO3)n+ H2O Cr OH HO O O Cr(VI) is used in dil. H2SO4, Ac2O, tBuOH or in pyridine. 2HCrO4 - H2O + Cr2O7 -- (CrO3)n + Ac2O Cr O O OCOCH3 H3COCO (CrO3)n + Me3COH Cr O O OCMe3 Me3CO (CrO3)n + Cr O O N+ - O N For formyl group
- 4. Jone’s reagent (CrO3)n + Cr O O N+ - O N CrO3 + dil. H2SO4 Collin’s reagent
- 5. Oxidation of hydrocarbon Need vigorous condition, mixture of product obtained with low yield In presence of O2, hydrocarbon burn and gives CO2 and H2O. In absence of activating hydroxyl or amino substituent, benzene rings are only slowly attack by chromic acid or permanganate. Used methyl ether or acetyl derivative of ring hydroxy and amino group respectively. Otherwise they activate the ring and ultimately quinones, CO2 and H2O may be obtained. Very resistant to oxidation
- 6. NO2 COOH NO2 Na2Cr2O7, aq. H2SO4 C8H17 CrO3, CH3COOH aq. H2SO4 COOH COOH O + Examples:
- 7. The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride.
- 15. Heterocyclic Chemistry: Quinoline, Isoquinoline and Indole A benzene ring can be fused on to the pyridine ring in two ways giving the important heterocycles quinoline, with the nitrogen atom next to the benzene ring, and isoquinoline, with the nitrogen atom in the other possible position. Indole itself has a benzene ring and a pyrrole ring sharing one double bond. It is an aromatic system with 10 electrons, eight from four double bonds and the lone pair from the nitrogen atom.
- 16. Several synthetic antimalarial drugs are based on the quinoline nucleus, chloroquine is an example. Ciprofloxacin is one of the several 4- quinolone-based antibiotics in use. The opium poppy alkaloids papaverine and in more-elaborated form, morphine are the examples.
- 17. Quinoline N N N O NH2 O O OR O A B Path - 1 Path - 2 Retero-synthetic disconnection approach: N NH2 O O NH2 + O o-aceyl aniline a ketone containing an -methylene group
- 18. We know formation of C-N bond is easier so we may consider the following dis-connection approach. Synthesis of quinoline; path – 1: Quinoline derivative N NH2 NH2 O O NH2 + O H H ,-unsaturated carbonyl compound o-aceyl aniline a ketone or aldehyde containing an -methylene group
- 19. Friedlander synthesis: Friedlander synthesis of quinoline derivative involves by using o-aceyl aniline and a ketone containing an -methylene group, the reaction can be carried out in presence of acid or alkali. Ph O NH2 CH3 O CH3 + N Ph Et N Ph Me Me AcOH, Trace H2SO4 KOH, EtOH In acid medium the reaction proceeds through the formation of more stable enol and in base medium the reaction occurs through the abstraction of more acidic hydrogen atom. A B
- 20. Synthesis of quinoline derivatives through path-A (base catalysed) Ph O NH2 + H2C O Et NH2 Et O Ph Aldol condensation and dehydration N Et OH Ph H N Et Ph A problem is found that the dimerisation of o-aceyl aniline, so that we should not take the following compound as starting material. NH2 O H2N O + N N
- 21. Pfitzinger Modification: The dimerisation problem was solved by Pfitzinger using ISATIN (A) as starting material which in alkaline medium gives compound B. That compound B will be used as the substrate of quinoline synthesis. N O O H NH2 COO- O A B Synthesis of Isatin: Isatin is commercially available. It may be prepared by cyclicizing the condensation product of chloral hydrate, aniline and hydroxylamine in sulfuric acid. This reaction is called the Sandmeyer isonitrosoacetanilide Isatin Synthesis and discovered by T. Sandmeyer in 1919. N O O H NH2 Cl Cl Cl OH OH N H Cl Cl HO OH N H N OH O Conc. H2SO4 NH2OH, HCl H2O
- 22. N O O H H3C + H3C O OPh NaOH, H3O+ N COOH OPh CH3 H3C N OPh CH3 H3C CaO, Pfitzinger modification towards the synthesis of quinoline derivative using isatin as starting material:
- 23. Synthesis of quinoline; path – 2: N N O NH2 O O OR O A B Procedure - A Procedure - B NH2 O + Skrup synthesis NH2 O O + Cambes Syntheisis Conrod-Limpach Knoor process (1, 3-Dicarbonyl) (-ketoester) (-ketoester)
- 24. Combes Synthesis: NH2 MeO MeO O O + N MeO MeO O N Me Me MeO MeO H+ Aromatic electrophilic substitution on highly activated aromatic nucleus Procedure - A Arylamine and 1,3-dicarbonyl are the substrates N MeO MeO O N Me Me MeO MeO N MeO MeO HO H H
- 25. In case of unsymmetrical ketone, we may get two products. Arylamine and -ketoester are the substrates. Conrod- Limpach Synthesis: KCP Knoor synthesis: TCP NH2 O O OEt + N OH O AcOH, 140o C N Me OH 260o C NH2 O O OEt + N OEt O AcOH, 40o C N OH Me 260o C
- 26. Procedure - B NH2 O + Skrup synthesis Skraup Synthesis: ,-unsaturated carbonyl and aniline NH2 O + Michael type condensation N H O N H N [O] Aromatic electrophilic substitution in highly activated nuclous
- 27. Skrup Synthesis for quinoline: This concept was used by Skrup towards the synthesis of quinoline by heating aniline, glycerol, nitrobenzene, H2SO4 and FeSO4. Step-1: Formation of acroline from glycerol by dehydration. ,-unsaturated aldehyde Step 2: Michael type condensation. NH2 O H + Michael type condensation N H OHC CH2OH CHOH CH2OH CH2OH CHOH2 CH2OH C CH2 CH2OH OH -H2O H H CHO CH2 CH2OH2 CHO CH CH2 -H2O H H-transfer H-transfer
- 28. Step 3: Aromatic electrophilic substitution on highly activated aromatic nucleus. N H N H OHC H2SO4 Dihydroquinoline Step 4: Oxidation of dihydroquinoline to quinoline. N H N PhNO2 Dihydroquinoline Quinoline FeSO4 is used to control the violence of the reaction. P-chloranil is the best oxidant. In this way we can prepare unsubstituted quinoline.
- 29. NH2 NO2 i. Glycerol, H2SO4, ii. As2O5 NO2 N MeO MeO Various oxidizing agent may be used such as, Fe2(SO4)3, SnCl4, As2O5 etc. Skrup synthesis is not applicable when acid sensitive groups are present in the benzene ring. In case of nitroaniline type compound we have to use another oxidizing agent particularly As2O5. Synthesis of derivatives of quinoline. N Me N Me
- 30. N Me N Me NH2 O H + H3C O H N Me N NH2 O H3C + H3C O H3C CH2=O Me2NH + +
- 31. Isoquinoline N Disconnection approach: Path-1 NH2 O O N N + NH2 + + A It is quite impossible to get compound A as it may easily polymerised. For this reason we have to take a blocked amino aldehyde, i.e. Compound B. NH2 EtO EtO Compound B OH Cl2 gas Cl CHO Excess EtOH Cl OEt OEt H2N OEt OEt NH3
- 32. Synthesis of derivatives of Isoquinoline CHO NH2 OEt OEt N OEt OEt N C. H2SO4 This reaction is known as Pomernz-Fritsch synthesis of isoquinoline derivative, where the starting materials are aromatic aldehyde and amino acetal. The reaction can also be applied when the benzene nucleus contain any type of substituents (electron donating or withdrawing). Obviously best result will be obtained when e-donating group is present in the aromatic ring. CHO NH2 OEt OEt N OEt OEt N C. H2SO4 Br Br Br
- 33. N R Amino acetal does not react with aromatic ketone. So it is difficult to get following type of products. N N R R R NH2 + O O Here also another problem will be arised. R NH2 + O O H H Ph R N N Ph R R NH2 O R NH2OH LiAlH4 CHO EtO OEt Disconnection approach: Path-2
- 35. N NH2 + O Cl Bishler’s Naperialski Reaction: Synthesis of isoquinoline derivatives NH2 CN Br CH3 NH O N H2N Ac2O POCl3 N [O] Disconnection approach: Path-3 A phenylethyl amine reacts with carboxylic acid chloride to form an amide, which can be cyclised with the loss of water to a 3,4-dihydroisoquinoline. Then it is readily dehydrogenated to isoquinoline derivative. The common cyclisation agents are P4O10, POCl3, PCl5.
- 36. Hence the electrophilic character is poor. It will only react with highly activated aromatic nucleus for the occurrence of the reaction, benzene nucleus should not contain any electron withdrawing group. Mechanism: NH Me O P Cl Cl Cl O NH Me O P Cl Cl Cl - O NH Me O P Cl Cl Cl- O NH Me O P Cl Cl O Cl N Me Cl H N H Cl Me H N Me -HCl N Pd/C 190o C
- 37. Benzene ring having e-withdrawing substituent Benzene ring having e-donating substituent: NH R O NO2 N NO2 R ~ 5 % POCl3 NH R O OMe N OMe R ~ 80 % POCl3
- 38. Reaction chemistry of quinoline and isoquinoline Under slightly acidic conditions quinolines and isoquinoline are susceptible to straight forward electrophilic substitution in the homocyclic ring. Reaction has been shown to involve the cations and the formal positive charge on nitrogen makes attack of the hetero ring very difficult. C-5-substitution occurs, not C-6 N H H X N H H X N H H X More stable Isoquinoline behaves like similar way
- 39. Benzene ring C-protonation, and hence exchange, via N-protonated quinoline, requires strong sulfuric acid and occurs fastest at C-8, then at C-5 and C-6; comparable exchange in isoquinoline takes place somewhat faster at C-5 than at C-8. At lower acid strengths each system undergoes exchange H atom to nitrogen, at C-2 for quinoline and C-1 for isoquinoline. These processes involve a zwitterion produced by deprotonation of the N-protonated heterocycle. N -H H N H N N H H H H H
- 40. Nitration: Quinoline gives approximately equal amounts of 5- and 8- nitroquinolines, whereas isoquinoline produces almost exclusively 5-nitro- isomer; mechanistically the substitutions involve nitronium ion attack on the N-protonated heterocycles. Sulfonation: Sulfonation of quinoline gives largely the 8-sulfonic acid whereas isoquinoline affords the 5-acid. Reactions at higher temperatures produce other isomers, both quinoline 8-sulfonic acid and quinoline 5-sulfonic acid are isomerised to the 6-acid under thermodynamic control. N N NO2 N NO2 + HNO3, C. H2SO4 0o C 1:1 N N SO3H 30% Oleum 90o C
- 41. Halogenation: In concentrated sulfuric acid, quinoline gives a mixture of 5- and 8-bromo derivatives; isoquinoline is efficiently converted into the 5-bromo derivative in the presence of aluminium chloride. N N N + Br Br Conc. H2SO4 Introduction of halogen to the hetero-rings occurs under remarkably mild conditions in which the nitrogen lone pair initiates a sequence by interaction with an electrophile. Thus treatment of quinoline and isoquinoline hydrochlorides with bromine produces 3-bromoquinoline and 4- bromoisoquinoline respectively as illustrated below for the latter.
- 42. Halogenation on heterocyclic ring: N N NO2 AcO-NO2 N N Br Br2/CCl4, RT N N Br2/CCl4, RT Br N N NO2 AcO-NO2
- 44. Hydroxylation: Both quinoline and isoquinoline can be directly hydroxylated with potassium hydroxide at high temperature with the evolution of hydrogen. 2-Quinolone ('carbostyril') and 1-isoquinolone ('isocarbostyril') are the isolated products.
- 45. Indole is an important heterocyclic system because it is built into proteins in the form of the amino acid tryptophan (Chapter 49), because it is the basis of important drugs such as indomethacin, and because it provides the skeleton of the indole alkaloids—biologically active compounds from plants including strychnine and LSD. clayden Indole
- 46. Electrophilic substitution: Pyrrole reacts with electrophiles at all positions but prefers the 2- and 5-positions, while indole much prefers the 3-position. In many ways the chemistry of indole is that of a reactive pyrrole ring with a relatively unreactive benzene ring standing on one side electrophilic substitution almost always occurs on the pyrrole ring. But indole and pyrrole differ in one important respect. In indole, electrophilic substitution is preferred in the 3-position with almost all reagents. Halogenation, nitration, sulfonation, Friedel–Crafts acylation, and alkylation all occur cleanly at that 3-position. This is, of course, the reverse of what happens with pyrrole. An explanation is that reaction at the 3-position simply involves the rather isolated enamine system in the five-membered ring and does not disturb the aromaticity of the benzene ring. The positive charge in the intermediate is delocalized round the benzene ring, but it gets its main stabilization from the nitrogen atom. It is not possible to get reaction in the 2-position without seriously disturbing the aromaticity of the benzene ring. 3-position 2-position
- 47. A simple example is the Vilsmeier formylation with DMF and POCl3, showing that indole has similar reactivity, if different regioselectivity, to pyrrole.
- 49. Nature is the master of molecular recognition and so this type of binding is in the heart of most biological processes. What is Molecular Recognition? The specific, non-covalent association between a receptor molecule and a particular substrate is primarily called molecular recognition. Whether it is an enzyme-substrate, antibody- antigen, or neuroreceptor-neurotransmitter pair, large molecules bind smaller one tightly and specifically.
- 50. K+ SCN- O O O O O O K+ O O N O O N O O I- Cl- Li+ O O O H3C CH3 CH3 CH3 H3C CH3 H3C CH3 CH3 O CH3 O H3C O CH3 Pedersen’s Model, 1967 Lehn’s Model, 1969 Cram’s Model, 1973 The seminal observation of Pedersen were brilliantly exploited by other workers most notably by D. J. Cram and J. M. Lehn and three shared the Nobel Prize in chemistry in 1987. Crown ether Cryptand Spherand
- 51. (2) Supramolecular assemblies, polymolecular entities, which is made by the spontaneous association of a large undefined number of components into a specific phase. The word ‘supramolecular chemistry’ has been used in particular for large multi-protein architectures and organized molecular assemblies. The term ‘supramolecular chemistry’ first used by Professor J.-M. Lehn, 1978. Supramolecular chemistry consists of two concerning areas: (1) Supermolecules, well-defined discrete oligomolecular species that result from the intermolecular association of a few component based on the principles of molecular recognition. What is Supramolecular Chemistry?
- 52. Molecular Recognition Depends on…… • Hydrogen Bonding Interactions • p-stacking Interactions • Electrostatic Force of Attractions • van der Waal’s Force • Hydrophobic Force
- 53. Molecular Recognition Depends on…… • Hydrogen Bonding Interactions Multiple hydrogen bonding a good way to achieve recognition because of its strength, directionality and specificity.
- 54. Molecular Recognition Depends on…… p-stacking Interactions Term introduced by E. J. Corey, 1972. Important in DNA and protein crystal structure. Face to Face, Edge to Face (T-shaped), slipped stack, CH-p stack are important. Slip stacked T-shaped stacked Stacked H
- 55. Molecular Recognition Depends on…… • Electrostatic Force of Attractions Ion-ion interactions Ion-dipole interactions Dipole-dipole interactions etc.
- 56. Molecular Recognition Depends on…… • van der Waal’s Force Polarisation of electron cloud by the close proximity of an adjacent nucleus.
- 57. Molecular Recognition Depends on…… • Hydrophobic Force Importance in the binding of organic guests by cyclodextrins and cyclophane hosts in water.
- 58. The designing Principle……… (i) Shape and size complementarities between the host and guest molecule i.e. convex and concave domains in the correct location (Host-convergent; Guest-Divergent). (ii) Large contact area between the receptor-substrate. (iii) Presence of complementary binding sites such as positive- negative, charge-dipole, dipole-dipole, hydrogen bonding donor-acceptor. (iv) Multiple interaction sites, which increase the association constant as the number of interaction sites increases. (v) The medium i.e. the solvent; the chance of hydrogen bond formation between solvent-substrate will be possible, if more polar solvent is used.
- 59. Application of Molecular Recognition: 1. Phase transfer agents. 2. Separation of mixture. 3. Molecular sensors. 4. Switches and molecular machinery 5. Catalyst 6. Pharmaceuticals 6.1 Anti-cancer agents 6.2 Anti-HIV activity 6.3 Drug design etc.
- 60. Application of Molecular Recognition: 1. Phase transfer agents 3 ] [ L L D D O NH Me Me O O Me O Me Me NH O Me Me O Valinomycin K+ + Cl - + F- + F Cl K+ [18]-crown-6 CH3CN KF Cl O O O O O O O O O O O O K+ K+ is complexed by oxygen atom of the antibiotic ester groups and once it is encapsulated within the macrocycle, it can be transported through the hydrophobic membrane. The lipophilic membrance will not normally allow charged species to pass through. The enterior of the K+-valinomycin complex, has peripheral alkyl groups that are ‘greasy’ providing the complex with solubility in the membrane and allowing transport of the metal ion. On the other hand, KF not soluble in acetonitrile and does not react with benzyl chloride i.e. F- cannot displace the Cl- atom. But when we use 18-crown-6, the reaction is quantitatively yielded to completion. The cause behind this is that 18-crown-6 encapsulated K+ and free F- displaces chlorine.
- 61. Application of Molecular Recognition: Chiral separation of mixture H3C H3C O O O O O CH3 O CH3 O Polymer (RR)-binapthyl crown ether bound to a polystyrene resin Cram and coworker have attached chiral crown ethers to polystyrene resin and used the resulting materials to get chiral chromatographic separations of amino acids and ester salts. Chiral substrates passing through a column, made of this material, interact with the crown ethers forming diastereomeric complexes, which have different stability constants. The R- entiomer forms a thermodynamically more stable complex with the host and is more strongly bound to the column. Therefore, the S-enantiomer emerges from the column first and resolution of the enantiomers is achieved. Chiral chromatographic separations are of great interest to the pharmaceutical industry, which often has to resolve two enantiomers of a chiral drug, which possess completely different activities
- 62. Application of Molecular Recognition: Molecular sensors R R G G Fluorescence sensing of chemical and biochemical analytes is an active area of research. This research is being driven to eliminate radioactive tracers, which are costly to use and to dispose of. There is a need for rapid and low-cost testing method for a wide range of chemical, bioprocess and environmental applications. Sensors should ideally be selective for a particular guest and not only report the presence of the guest molecule, but should also allow the chemist to monitor its concentration. This quantitative analysis by fluorescence sensor is medically and environmentally important.
- 63. Switches and molecular machinery technology use individual molecules for controlling and storing information, acting as ‘on-off switches’ and ‘logic gates’. If the molecule contain an electron donor group like amine, then the phenomenon will be more exciting. This phenomenon has been exploited to develop sensors bound on quenching of polynuclear aromatic hydrocarbons by amines. The basic idea is that quenching by amines requires that the lone pair is bound to a proton, then electron transfer is inhibited and the fluorescence is not quenched. Such probes are said to undergo photoinduced electron transfer from the nitrogen into the aromatic ring. Preventing PET, in similar way the emission from the anthracene unit is observed. So protons switch on the fluorescence. H + N O Cl No PET fluorescent Not fluorescent PET N O Cl H+ Moleculplication of Molecular Recognition: ar ‘on-off’ switch Switches and molecular machinery
- 64. Catalyst + ADP 2 NH O HN N PO3 NH O HN HN 2 O P O P O P O adenosine O O O O O O H O H H H H NH NH N NH NH O NH H Protonated macrocyclic polyamines can catalyse the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP). Cyclic hexaammonium cation strongly binds ATP and an intramolecular transfer of the terminal phosphate to the sixth nitrogen gives an intermediate, which then loses phosphate ion by reaction with water.
- 65. Pharmaceuticals: Anti-cancer agents HO HO OMe O O OMe O O O O N N N N N OAc AcO Gd(III) Texaphyrin metal complexes of Gd-Tex Texaphyrin is an expanded porphyrin synthesized by Sessler et al. The gadolinium complex Gd-Tex is currently undergoing trials for use as a radiation sensitizer. A cancer patient is exposed to radiation. When Gd-Tex is exposed to ionising radiation in vivo, it captures an electron and becomes a p- radical cation.
- 66. spacer NH HN N HN HN HN HN N Anti-HIV activity Bicyclam, a receptor for two transition metal cations, exhibits potent inhibition of the HIV virous at early stage in its replication cycle. It is possible that this inhibition is mediated by transition metals. Linked bicyclam
- 67. N N O O H H HO OH O H H N N O O lle 50' lle 50 Asp 25' Asp 25 Drug design is an iterative process, which begins when a chemist identifies a compound that displays an interesting biological profile and ends when both the activity profile and the chemical synthesis of the new chemical entity are optimized. Traditional approaches to drug discovery rely on a step-wise synthesis and screening program for large numbers of compounds to optimize activity profiles. Over the past ten to twenty years, scientists have used computer models of new chemical entities to help define activity profiles, geometries and reactivity. Drug design Inhibitor for HIV protease bound in the enzyme active site