Organic Chemistry 4.pptx
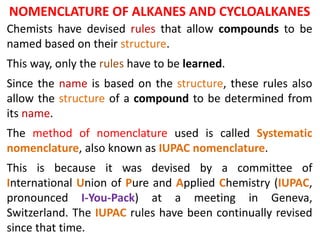
- 1. Chemists have devised rules that allow compounds to be named based on their structure. This way, only the rules have to be learned. Since the name is based on the structure, these rules also allow the structure of a compound to be determined from its name. The method of nomenclature used is called Systematic nomenclature, also known as IUPAC nomenclature. This is because it was devised by a committee of International Union of Pure and Applied Chemistry (IUPAC, pronounced I-You-Pack) at a meeting in Geneva, Switzerland. The IUPAC rules have been continually revised since that time. NOMENCLATURE OF ALKANES AND CYCLOALKANES
- 2. Unbranched alkanes named (after the first four) according to the number of carbon atoms present, that is, the Greek Latin roots, such as pent-, hex-, hept-, oct-, etc. are used to designate the length of the chain. The prefix “n” before the name stands for normal to emphasize that the carbon atoms are in a continuous chain. Some examples are: Names such as isobutane and neopentane, are non- systematic and are called common names. Unbranched Alkanes The names of alkanes have the ending “ane”.
- 3. Some Examples of Unbranched Alkanes
- 4. substituent substituent substituent Branched Alkanes For branched-chain alkanes, the longest continuous chain of carbon atoms determines the name of the alkane. Some examples are: Hexane Pentane The groups attached to the main (parent) chain are called substituents. The main (parent) chain is numbered so as to give the lowest number to the carbon atom with the first substituent encountered along the chain. Some examples are: CH3CH2CH2CH2CH CH3 CH3 CH3CHCHCH2CH3 CH3 CH3
- 5. CH3CH2CH2CH2CHCH3 CH3 1 2 3 4 5 6 CH3CHCHCH2CH3 CH3 CH3 1 2 3 4 5 If a single hydrogen is removed from an alkane, an alkyl group is obtained. The name of the alkyl group is obtained by replacing the “ane” ending of the alkane with “yl”. Some examples are: Alkane name Formula of alkane Formula of alkyl group Alkyl group name Methane CH4 CH3- Methyl Ethane CH3CH3 CH3CH2- Ethyl Propane CH3CH2CH3 CH3CH2CH2- n-Propyl The letter “R” is used to indicate an alkyl group.
- 6. The number allocated to the carbon atom having the substituent is used to designate the location of the substituent group. The numbers designating the locations of the substituent groups are separated from each other by a comma, while they themselves are separated from words by a hyphen. Some examples are: The name obtained after combining all the different aspects has three parts. Number designating the location of the substituent group along the main chain (locant). The substituent group (prefix). The parent (main) name.
- 7. When two or more substituents are present, each substituent is given a number corresponding to its location on the longest continuous chain of carbon atoms. The substituent groups are listed alphabetically in the name. For example: CH3CH2CH2CH2CHCH3 CH3 1 2 3 4 5 6 2-Methylhexane CH3CH2CHCH2CH2CH3 CH2 CH3 5 4 3 2 1 6 CH3CH2CH2CH2CHCH3 CH2 CH3 1 3 4 5 6 7 2 3-Methylheptane 3-Ethylhexane
- 8. When two or more substituents are identical, the prefixes di-, tri-, tetra-, etc. are used to indicate this. For example: 4-ethyl-2-methylhexane CH3CH CH2CH CH2CH3 CH2 CH3 CH3 5 4 3 2 1 6 CH3CHCHCH2CH3 CH3 CH3 1 2 3 4 5 2,3-Dimethylpentane CH3CHCHCH3 CH3 CH3 1 2 3 4 2,3-Dimethylbutane When two substituents are present on the same carbon atom, the number indicating the location of the substituents is used twice. For example:
- 9. When chains of equal length compete for selection as the parent chain, the chain with the greater number of substituents is chosen. For example: 3-Ethyl-3-methylhexane CH3CH2C CH2CH2 CH2 CH3 CH3 CH3 2,3,5-Trimethyl-4-propylheptane (4 substituents) Not 4-sec-Butyl-2,3-dimethylheptane (3 substituents) CH3 CH2 CH CH CH CH CH3 CH3 CH3 CH3 CH2CH2CH3 5 4 3 2 1 6 7 1 2 3 4 5 6 7
- 10. When branching first occurs at an equal distance from either end of the longest chain, the name that gives the lower number at first point of difference is chosen. For example: 2,3,5-Trimethylhexane Not 2,4,5-trimethylhexane Using the upper numbering, the name is 2,3,5- trimethylhexane. When the lower numbering is used, the name is 2,4,5-trimethylhexane. The correct name is 2,3,5- trimethylhexane. CH3 CH CH2 CH CH CH3 CH3 CH3 CH3 5 4 3 2 1 6 1 2 3 4 5 6
- 11. If the same substituent numbers are obtained in both directions, the first cited group (alphabetically) receives the lower number. An example is: 3-Ethyl-5-methylheptane Not 5-Ethyl-3-methylheptane CH3 CH2 CH CH2 CH CH2 CH3 CH2 CH3 CH3 5 4 3 2 1 6 7 1 2 3 4 5 6 7
- 12. Alkyl Substituents We observed that alkyl groups or substituents are named by replacing “ane” ending of the alkane with “yl”. Two alkyl groups – a propyl and an isopropyl – contain three carbon atoms. A propyl group is obtained when a hydrogen atom is removed from a primary carbon of propane. A primary carbon is one that is bonded to only one other carbon. It has three hydrogen atoms bonded to it. These three hydrogen atoms are called primary hydrogen atoms. Primary is denoted by 1˚.
- 13. Alkyl Substituents … An isopropyl group is obtained when a hydrogen atom is removed from a secondary carbon of propane. A secondary carbon is one that is bonded to two other carbon atoms. It has two hydrogen atoms bonded to it. These two hydrogen atoms are called secondary hydrogen atoms. Secondary is denoted by 2˚.
- 14. a propyl group There are four alkyl groups that contain four carbon atoms. Butyl and isobutyl groups result from removal of a primary hydrogen atom from a primary carbon atom. A sec-butyl is obtained by removal of a hydrogen atom from a secondary carbon atom. Sec- often abbreviated s- stands for secondary. tert-butyl group has had a hydrogen removed from a tertiary carbon (tert- offen abbreviated t-, stands for tertiary). an isopropyl group CH3CH2CH2 A primary carbon CH3CHCH3 A secondary carbon
- 15. A tertiary carbon atom is a carbon that is bonded to three other carbon atoms. It has only one hydrogen atom bonded to it. This hydrogen atom is called a tertiary hydrogen atom. Tertiary is denoted by 3°. an n-butyl group an isobutyl group a sec-butyl group a tert-butyl group CH3CH2CH2CH2 CH3CHCH2 CH3 CH3CH2CH CH3 CH3C CH3 CH3
- 16. A carbon atom bonded to four other carbon atoms is called a quaternary carbon atom. It has no hydrogen atoms bonded to it. Alkyl group names are used so frequently that it is important to learn them. The most commonly used groups are listed below:
- 17. Nomenclature of small alkyl groups Group structure Name CH3CH─ │ CH3 Isopropyl or (1-methylethyl) CH3CHCH2─ │ CH3 Isobutyl or (2-methylpropyl) CH3CH2CH─ │ CH3 sec-butyl or s-butyl (1-methypropyl) CH3 │ CH3─C─ │ CH3 tert-butyl or t-butyl (1,1-dimethylethyl) CH3CHCH2CH2─ │ CH3 Isopentyl (3-methylbutyl) CH3 │ CH3─C─CH2─ │ CH3 Neopentyl (2,2-dimethylpropyl)
- 18. Names such as isopropyl, sec-butyl, and tert-butyl are acceptable substituent names in IUPAC system of nomenclature, but systematic substituent names are preferable. Systematic names are obtained by numbering the substituent beginning at the carbon that is bonded to the parent hydrocarbon. Some examples are: CH3CH2CH2─ n-propyl group CH3CH2CH3 1 2 CH3CHCH3 (isopropyl group) │ 1-methylethyl group CH3CH2CH2CH2─ n-butyl group CH3CH2CH2CH3 1 2 3 CH3CHCH2CH3 (sec-butyl group) │ 1-methylpropyl group
- 19. Examples of nomenclature of alkanes containing such groups are: CH3 │ CH3CHCH2─ (isobutyl group) 3 2 1 2-methylpropyl group CH3CHCH3 │ CH3 CH3 2 1│ (isobutane) CH3CCH3 (tert-butyl group) │ 2-methylpropane 1,1-dimethylethyl group 4-(1-Methylethyl)heptane 4-isopropylheptane heptane (parent chain) 1-methylethyl (substituent) OR CH3CH2CH2CH CH2CH2CH3 CH CH3 CH3 5 4 3 2 1 6 7 1 2
- 20. In this name, the substituent is in brackets; the number inside the brackets indicates a position on the substituent, whereas the number outside the brackets indicates a position on the parent hydrocarbon. 4-(1,1-Dimethylethyl)octane 4-tert-Butyloctane octane (parent chain) 1,1-dimethylethyl (substituent) CH3CH2CH2CH CH2CH2CH2 C CH3 CH3 CH3 CH3 5 4 3 2 1 6 7 8 1 2 OR
- 21. The prefix “cyclo” is used before the name of the cyclic parent alkane. Some examples are: Cyclopropane Cyclobutane Cyclopentane Cyclohexane Monocyclic Alkanes C H2 C H2 CH2 OR OR OR C H2 CH2 CH2 C H2 C H2 C H2 C H2 CH2 CH2
- 22. When the cyclic compound has a substituent, then the name of the substituent group occurs as a prefix. Some examples are: Methylcyclopropane Ethylcyclohexane n-Propylcyclobutane When two substituents are present, the ring is numbered beginning with the substituent first in the alphabet, and the direction of the numbering is continued so as to give the next substituent the lower number possible. Some examples are: CH3 CH2CH3 CH2CH2 CH3
- 23. When three or more substituents are present, numbering is done beginning at the substituent that leads to the lowest set of locants. Some examples are: CH3 CH2CH3 CH3 CH3 CH3 C H3 1-Ethyl-3-methylcyclohexane Not 1-ethyl-5-methylcyclohexane 1,2-Dimethylcyclopentane Not 1,5-dimethycyclopentane 1,1-Dimethylcyclopentane
- 24. The ring is rigid and rotation about the carbon-carbon bonds is not possible, its structure is fixed in space. Two ways of arranging two substituents on the ring are possible. For example: CH3 CH2CH3 CH3 Cl CH2CH3 CH3 2-Ethyl-1,4-dimethylcyclohexane 4-Chloro-2-ethyl-1-methylcyclohexane
- 25. When the two groups are on the same side, the prefix “cis” (Latin word for “on the same side”) is used. If the two groups are on opposite sides, the prefix “trans” (Latin word for “across”) is used. The two isomers are called geometric isomers. They have different physical properties. For example: CH3 CH3 H H CH3 H H CH3
- 26. cis-1,2-Dimethylcyclopentane trans-1,2-Dimethylcyclopentane bp 99˚C mp -62˚C bp 92˚C mp -120˚C CH3 H CH3 H CH3 H CH3 H CH3 H H CH3 CH3 H H CH3
- 27. Bridged Bicycloalkanes Bridged bicyclic alkanes have two rings that share two carbon atoms. For example: CH2 CH2 C H C H C H2 C H2 CH2 2-carbon bridge 1 carbon bridge bridgehead carbon atom OR OR
- 28. Numbering begins at a bridgehead along the longest chain (bridge), then back to the first bridgehead by the second longest chain. The shortest chain (bridge) is then numbered progressively starting from the first bridgehead. The number of carbon atoms forming each chain (bridge) are indicated in square brackets starting from the longest chain to the shortest chain. Some examples are: 1 2 3 4 5 6 7 Bicyclo[2.2.1]heptane OR Norbornane CH3 1 2 3 4 5 6 7 8 8-Methylbicyclo[3.2.1]octane 1 2 3 4 Bicyclo[1.1.0]butane