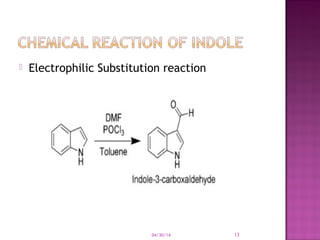
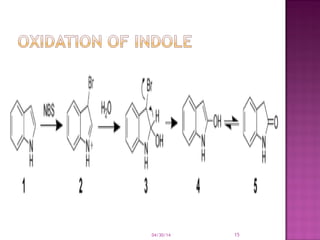
Heterocyclic compounds contain rings with at least two different elements, with one usually being carbon. They can be classified based on ring size, with common 5-membered rings including furan, thiophene, and pyrrole, and common 6-membered rings including pyridine and pyran. Many heterocycles like pyrrole are aromatic due to resonance stabilization. Rings are involved in various reactions like electrophilic substitution and oxidation.