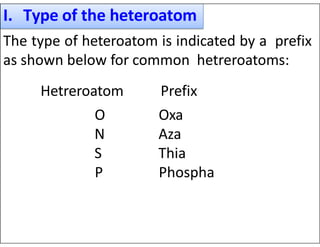
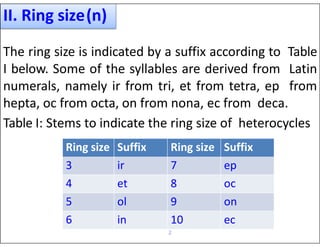
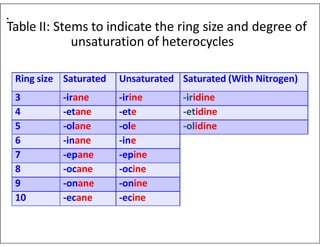
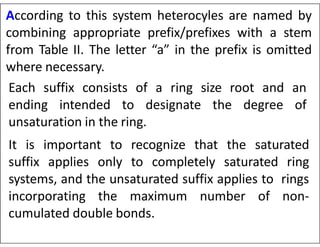
This document outlines the nomenclature system for naming heterocyclic compounds. It discusses:
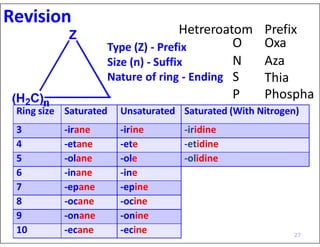
- Using prefixes to indicate the heteroatom type (e.g. oxa, aza) and suffixes for the ring size and degree of unsaturation.
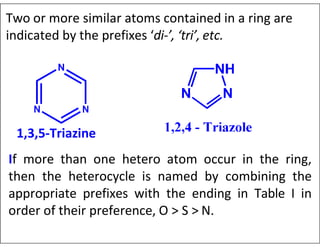
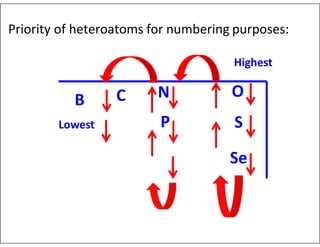
- Numbering the heteroatoms in rings with multiple heteroatoms from the most preferred (oxygen) to the least (boron).
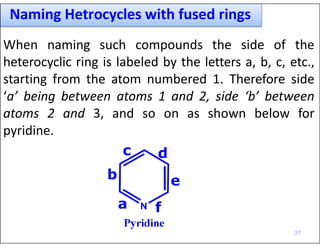
- Naming systems with fused rings by labeling the sides and using a prefix to indicate the additional ring.
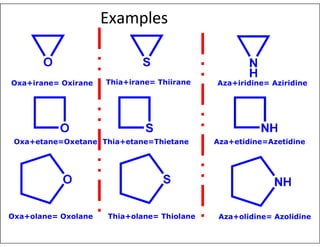
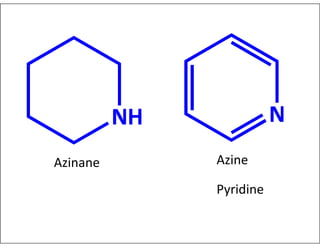
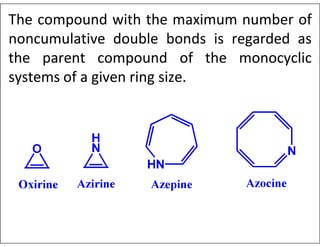
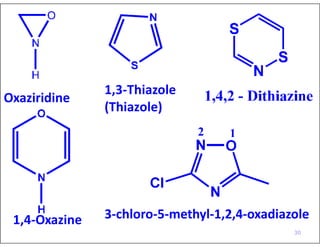
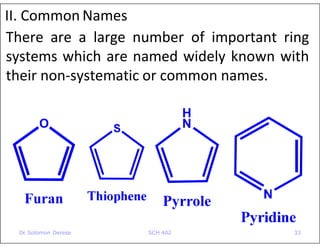
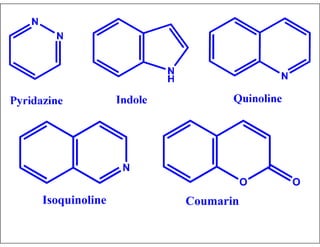
- Examples of common heterocyclic ring systems and their non-systematic names.
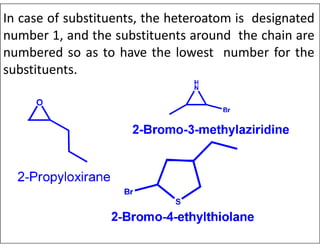
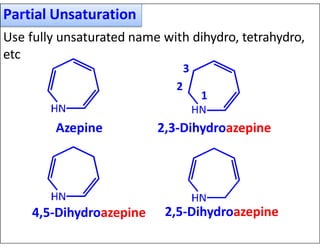
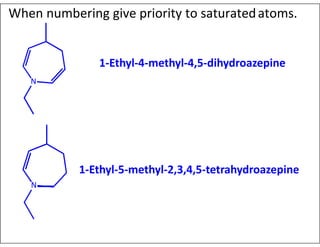
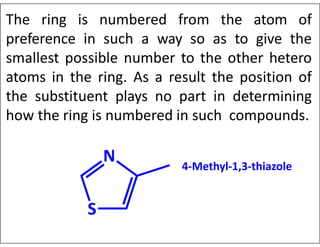
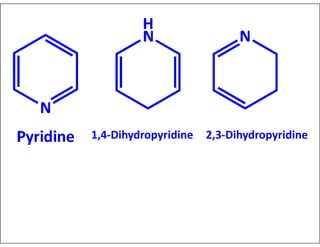
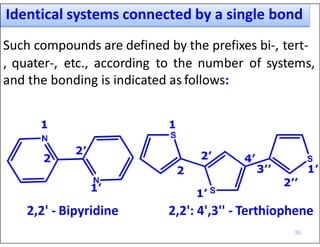
- Indicating partial unsaturation and multiple connected rings in names.



![The name of the heterocyclic ring is chosen as
the parent compound and the name of the
fused ring is attached as a prefix. The prefix in
such names has the ending ‘o’, i.e., benzo,
naphtho and so on.
Benzo [c] thiophene
Benzo [b] pyridine
a
Benzo [b] furan
b b c
a
a b
38](https://image.slidesharecdn.com/chapter1pocpresentationno3-201015092444/85/Chapter-1-poc-presentation-no-3-24-320.jpg)
![c
a
b
d Benzo [d] thiepine
39](https://image.slidesharecdn.com/chapter1pocpresentationno3-201015092444/85/Chapter-1-poc-presentation-no-3-25-320.jpg)

