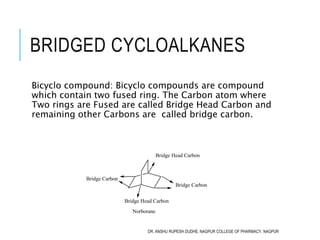
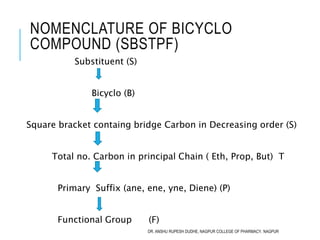
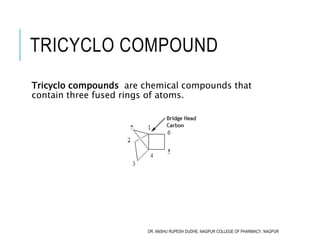
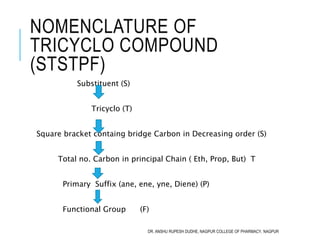
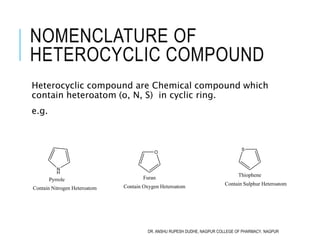
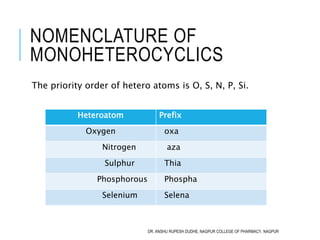
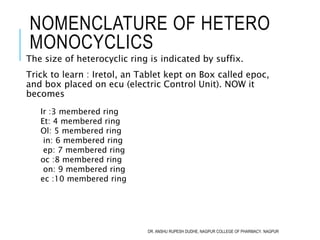
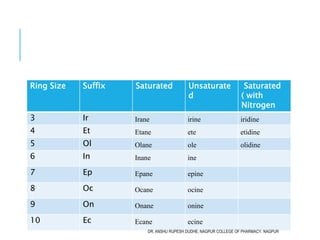
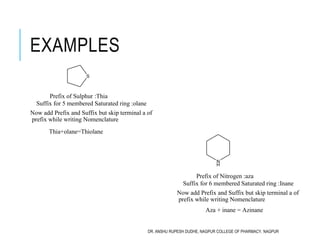
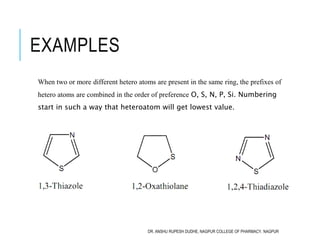
This document discusses the nomenclature of bridged cycloalkanes, heterocyclic compounds, and fused ring systems. It begins by explaining the nomenclature rules for bicyclo and tricyclo compounds using the SBSTPF and STSTPF systems respectively. It then discusses spiro and bridged heterocyclic compounds. The document provides numerous examples of applying these nomenclature rules. It also discusses the nomenclature of monoheterocyclic, fused heterocyclic, and heterocyclic compounds fused with benzene rings using appropriate prefixes and suffixes.

![EXAMPLES
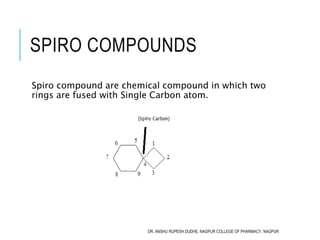
Largest Ring is preferred over Smallest ring. So
Numbering Start from Bridge head Carbon and Cover
Largest ring first
1
2
3
4
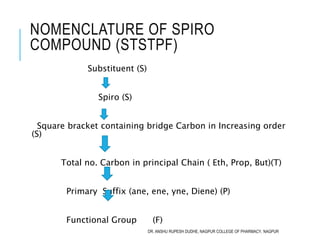
Rule: SBSTPF
Substituent: NIL
Bicyclo : Bicyclo
Square Bracket: [1.1]
Total No of Carbon : 4 ( but)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: Bicyclo[1.1]butane
Rule: SBSTPF
Substituent: NIL
Bicyclo : Bicyclo
Square Bracket: [4.2.0]
Total No of Carbon : 8 (oct)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: Bicyclo[4.2.0]octane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-4-320.jpg)
![EXAMPLES
CH3
CH3
1 2
3
4
5
6
7
8
9
Rule: SBSTPF
Substituent: Methyl Group
Bicyclo : Bicyclo
Square Bracket containing Bridge Carbon : [3.3.1]
Total No of Carbon containing Bridge carbon : 9 (Non)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: 1,3-DimethylBicyclo[3.3.1]nonane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-5-320.jpg)
![EXAMPLES
Rule: SBSTPF
Ring Substituent: Nitrogen (aza), Oxygen (Oxa)
Bicyclo : Bicyclo
Square Bracket containing Bridge Carbon : [3.2.0]
Total No of Carbon containing Bridge carbon : 7
(Hept)
Primary Suffix : ane
Function Group: Ketone (suffix: one)
IUPAC Name: 4-oxa-1-azaBicyclo[3.2.0]heptane
N
O
O 1 2
3
4
5
6
7
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-6-320.jpg)
![EXAMPLES
N
O
S
1
2
3
4
5
6
7
8
Rule: SBSTPF
Ring Substituent: Nitrogen (aza), Sulphur (Thia)
Bicyclo : Bicyclo
Square Bracket containing Bridge Carbon : [4.2.0]
Total No of Carbon containing Bridge carbon : 8 (Oct)
Primary Suffix : ane
Function Group: Ketone (suffix: one)
IUPAC Name: 5-Thia-1-azaBicyclo[4.2.0]octan-8-one
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-7-320.jpg)
![EXAMPLES
Rule: STSTPF
Substituent: NIL
Bicyclo : Tricyclo
Square Bracket containing Bridge Carbon : [2.2.1.0]
Total No of Carbon containing Bridge carbon : 7 (hept)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: Tricyclo[2.2.1.0]heptane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-10-320.jpg)
![EXAMPLES Br
1 2
3
4
5
6
7
8
9
Rule: STSTPF
Substituent: Bromo
Bicyclo : Tricyclo
Square Bracket containing Bridge Carbon : [3.2.2.0]
Total No of Carbon containing Bridge carbon : 9 (non)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: 2-BromoTricyclo[3.2.2.0]heptane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-11-320.jpg)
![Numbering start from one carbon away than
Spiro Carbon . Smallest ring is preferred over
largest ring.
4
5
6
7
8 9
1
2
3
Rule: SSSTPF
Substituent: NIL
Bicyclo : Spiro
Square Bracket containing Bridge Carbon : [3.5]
Total No of Carbon containing Bridge carbon : 9 (non)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: spiro[3.5]nonane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-14-320.jpg)
![EXAMPLES
4
5
6
7
8 9
3
2
CH3
1
Rule: SSSTPF
Substituent: Methyl
Bicyclo : Spiro
Square Bracket containing Bridge Carbon : [3.5]
Total No of Carbon containing Bridge carbon : 9 (non)
Primary Suffix : ane
Function Group: NIL
IUPAC Name: 1-methyl spiro[3.5] nonane
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-15-320.jpg)
![EXAMPLES
1
2
3
5
6
8
9 10
7 4
Rule: SSSTPF
Substituent: NIL
Bicyclo : Spiro
Square Bracket containing Bridge Carbon : [4.5]
Total No of Carbon containing Bridge carbon : 10 (dec)
Primary Suffix : diene
Function Group: NIL
IUPAC Name: 1-methyl spiro[4.5]dec-1,6-diene
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-16-320.jpg)


![NOMENCLATURE OF
HETEROCYCLIC RING FUSED
WITH BENZENE RING
Benzene ring is considered as Prefix and named as Benzo
and Heterocyclic ring is used as Suffix. Both Suffix and
Prefix are connected by Fused Letter in Square Bracket.
Prefix[Fusion Letter]Suffix
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-25-320.jpg)
![EXAMPLES
O
Benzo [b] furan
or Benzofuran
1
2
3
4
5
6
7
a
b
N
1
2
3
4
5
6
7
8
a
b
Benzo[b]pyridine
or Quinoline
S
1
2
3
4
5
6 7
a
b
c
Benzo[c]thiophene
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-26-320.jpg)
![NOMENCLATURE OF HETEROCYCLIC FUSED WITH
OTHER HETEROCYCLIC
Rules:
1. Name of minor ring or prefix [fusion number, fusion no. letter] name of
major ring or Suffix.
2Divide two rings. Numbered each Ring Individually.
3.Fusion letter comes from major ring and fusion no comes from minor ring.
4.Priority order: N, F, Cl, Br, I, O, S, Se (Heteroatom Nitrogen is preferred
over O or S).
5. A heterocyclic ring containing largest ring is preferred over smallest ring .
6. A heterocyclic ring containing greater no. of heteroatom and greater
variety of heteroatom is preferred
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-27-320.jpg)

![EXAMPLES
O
O
O
Considered as Major Ring because
large ring is preferred over small Ring
having simillar heteroatom
O
1
2
3
4
5
4
1
5
4
3
2
2H-Furo[3,2-b]pyran
1
2
3
4
5
6
7
a
b
Divide fused ring in to individual Ring.
Fusion letter comes from major ring and
fusion no comes from minor ring
Considered as Minor Ring due to small Ring
size.Fusion No. will be done according to Direction of
Major ring.
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-29-320.jpg)
![EXAMPLES
C o n s i d e r e d a s M a j o r R i n g
d u e t o g r e a t e r v a r i e t y
D i v i d e f u s e d r i n g i n t o i n d i v i d u a l R i n g . F u s i o n
l e t t e r c o m e s f r o m m a j o r r i n g a n d f u s i o n n o c o m e s
f r o m m i n o r r i n g
N
O
N
1
2
3
4
5
6
7
8
5 H - P y r i d o [ 2 , 3 - d ] o x a z i n e
a
b c
d
N
O
N
C o n s i d e r e d a s M i n o r R i n g d u e t o l e s s e r
v a r i e t y
1
2
3
1
2
3
4
5
6 4
5
6
a
b
c
d
e
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-30-320.jpg)
![EXAMPLES
N
N
N
N
Pyrazino[2,3-d]pyridazine
N
N
N
N
1
2
3
4
5
6
a
b c
d
1
2
3
4
5
6
Considered as M inor Ring due to greater Locant
Position.Fusion No. will be calculated according
to Direction of M ajor ring.
Considered as M ajor Ring due to Lower
Locant position.
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-31-320.jpg)
![EXAMPLES
S
N
N
1
2
3 a
b
Im idazo[2,1-b]thiazole
N
H
N
N
S
C onsidered as M ajor R ing due to greater variety.
C onsidered as M inor R ing due to
lesser variety
F usion N o. w ill be done according to
D irection of M ajor ring.
D ivide M olecule into tw o parts and com m on
atom is P art of both ring
1
2
3
4
5
a
b
c
d
1
2
3
4
5
DR. ANSHU RUPESH DUDHE, NAGPUR COLLEGE OF PHARMACY, NAGPUR](https://image.slidesharecdn.com/pptnomenclature-230113093616-26b2d3a2/85/PPT-nomenclature-pptx-32-320.jpg)