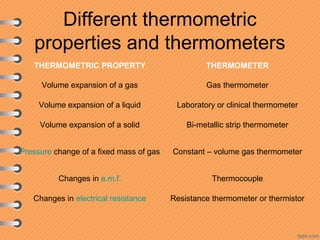
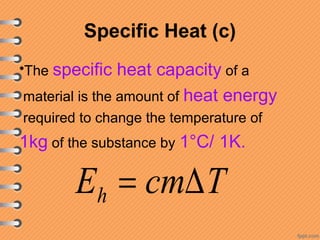
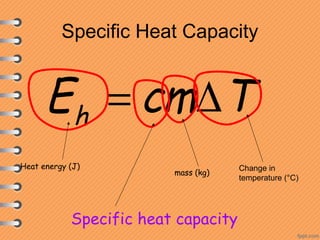
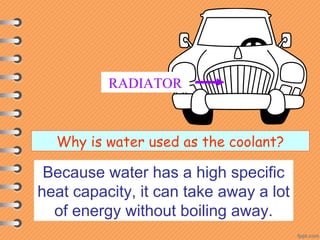
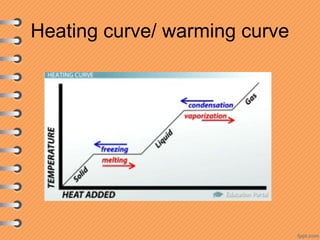
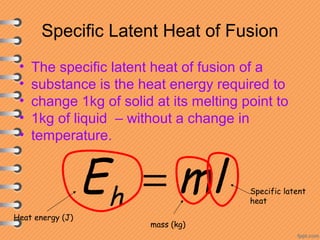
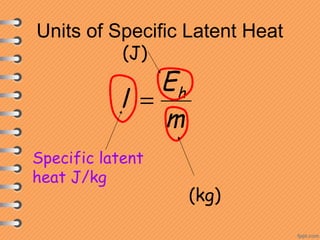
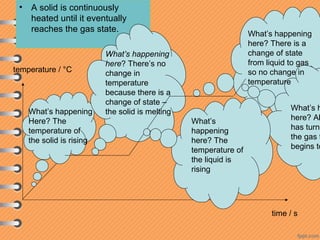
This document discusses heat and energy transfer. It explains the kinetic molecular theory of matter and how heat and temperature differ. Heat is the transfer of energy between objects due to a temperature difference, while temperature is a measure of the average kinetic energy of a substance's particles. The document also covers concepts like specific heat capacity, which is the energy required to change an object's temperature, and latent heat of fusion/vaporization, which is the energy absorbed during phase changes with no temperature change. Heat transfer occurs through conduction, convection and radiation.