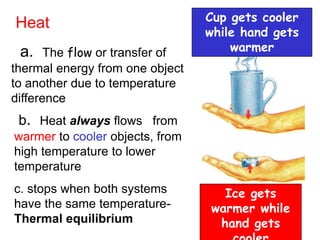
The document discusses the concepts of temperature and heat, including their definitions, measurement units, and the relationship between them in thermodynamics. It explains concepts such as the conservation of energy, heat transfer processes, the first and second laws of thermodynamics, and details on heat engines and heat pumps. Additionally, it covers the specific heat of materials, methods for calculating thermal energy changes, and key differences between heat and temperature.