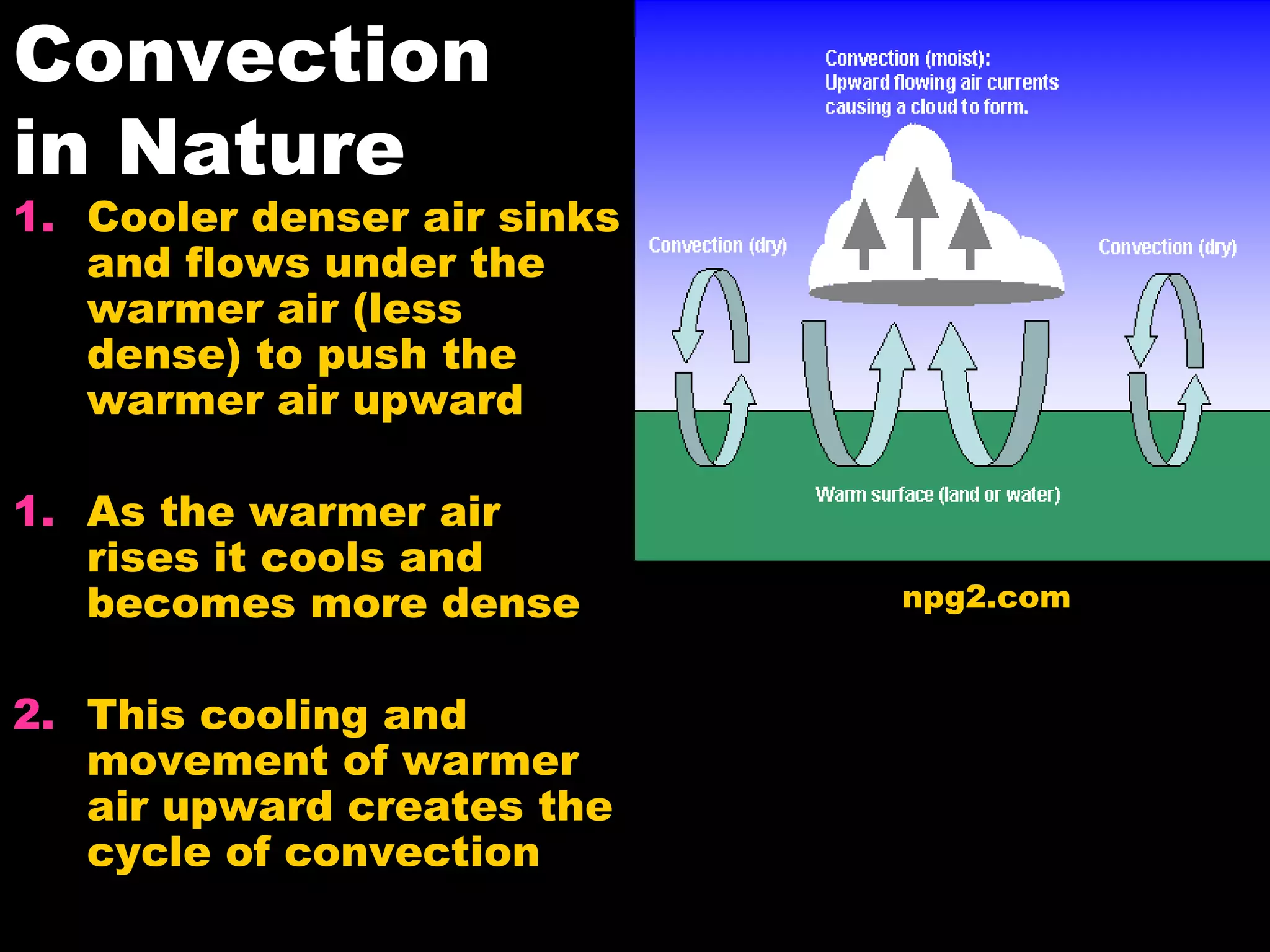
The document discusses heat, temperature, and the transfer of thermal energy. It defines heat as the flow of energy due to temperature differences and explains that temperature depends on the motion of particles. All matter is made up of atoms that are constantly moving, even in solids. Temperature is a measure of the average kinetic energy of particles. Thermometers can measure temperature because substances inside expand or contract with changes in temperature. Heat transfers between objects by conduction, convection, and radiation. Conduction involves direct contact, convection involves particle movement in fluids, and radiation involves electromagnetic waves.