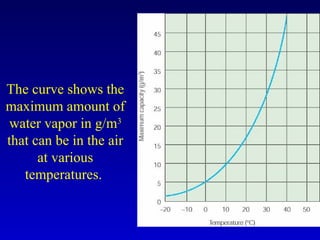
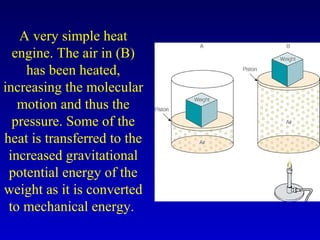
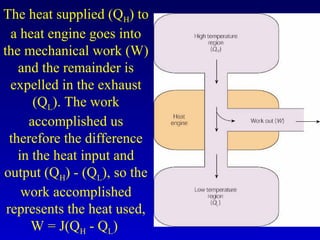
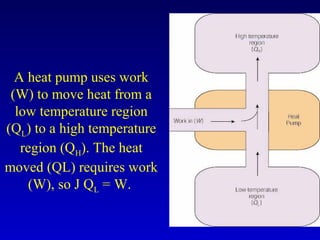
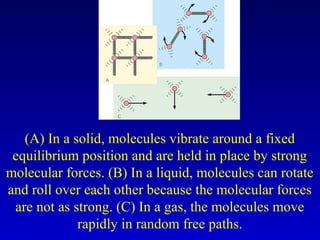
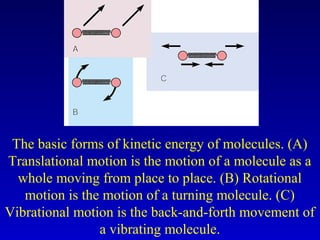
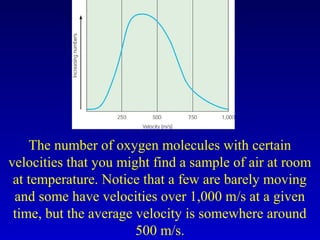
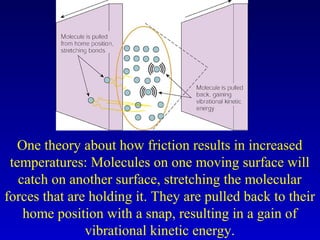
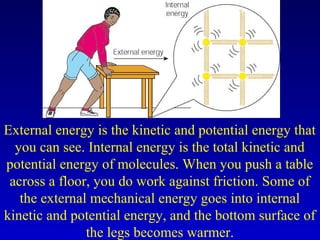
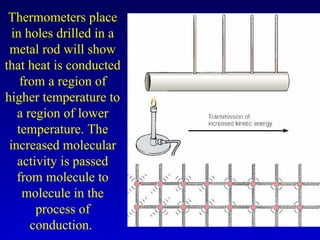
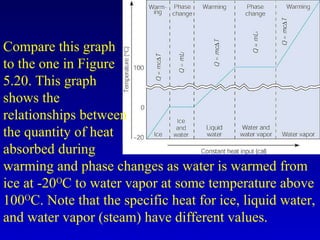
- Heat is the transfer of internal energy between objects due to a temperature difference. Temperature is a measure of the average kinetic energy of particles in an object.
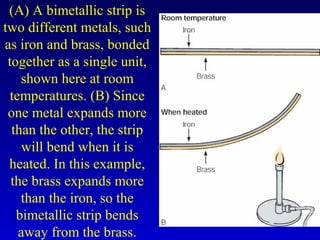
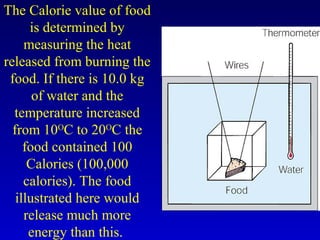
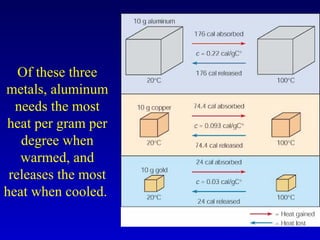
- Specific heat is the amount of energy needed to raise the temperature of 1 gram of a substance by 1 degree Celsius and depends on the substance. The amount of heat transferred depends on the mass, specific heat, and change in temperature.
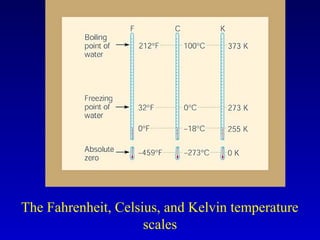
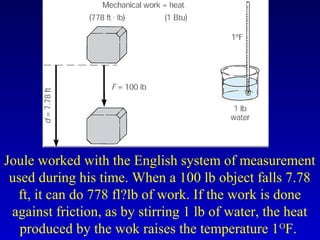
- Common units for heat include joules, calories, kilocalories, and British Thermal Units (BTUs). Conversions between temperature scales like Celsius, Fahrenheit and Kelvin are also discussed.




































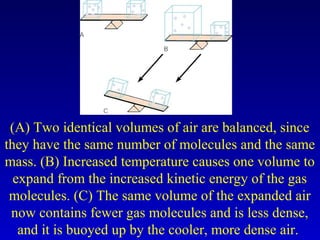














![Temperature is associated
with the average energy
of the molecules of a
substance. These numbered
circles represent arbitrary
levels of molecular kinetic energy that, in turn,
represent temperature. The two molecules with the
higher kinetic values [25 in (A)] escape, which lowers
the average value from 11.5 to 8.1 (B). Thus
evaporation of water molecules with more kinetic
energy contributes to the cooling effect of evaporation
in addition to the absorption of latent heat.](https://image.slidesharecdn.com/ch4-heatandtemperature-140806095120-phpapp02/85/Ch4-heat-and-temperature-68-320.jpg)