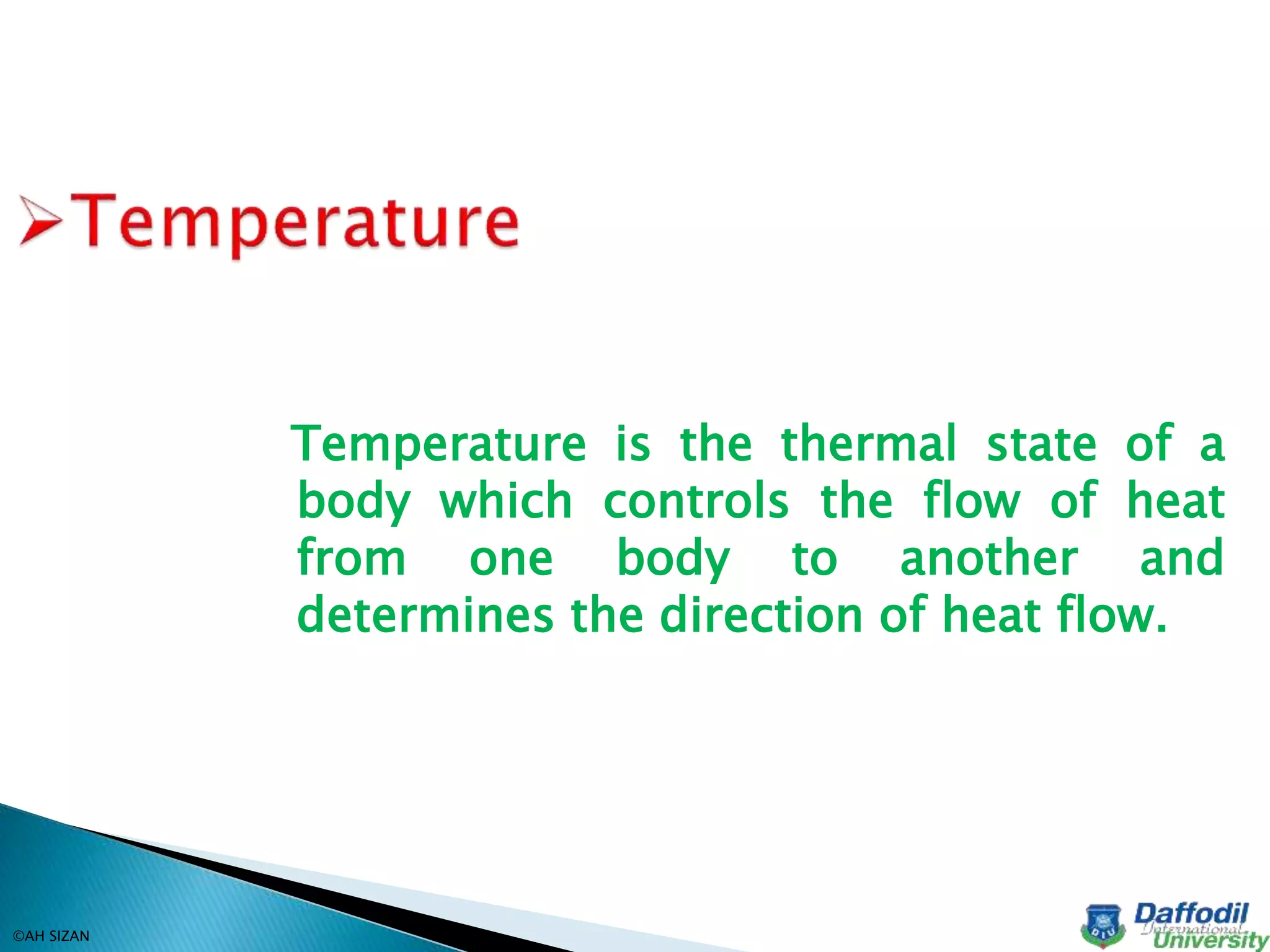
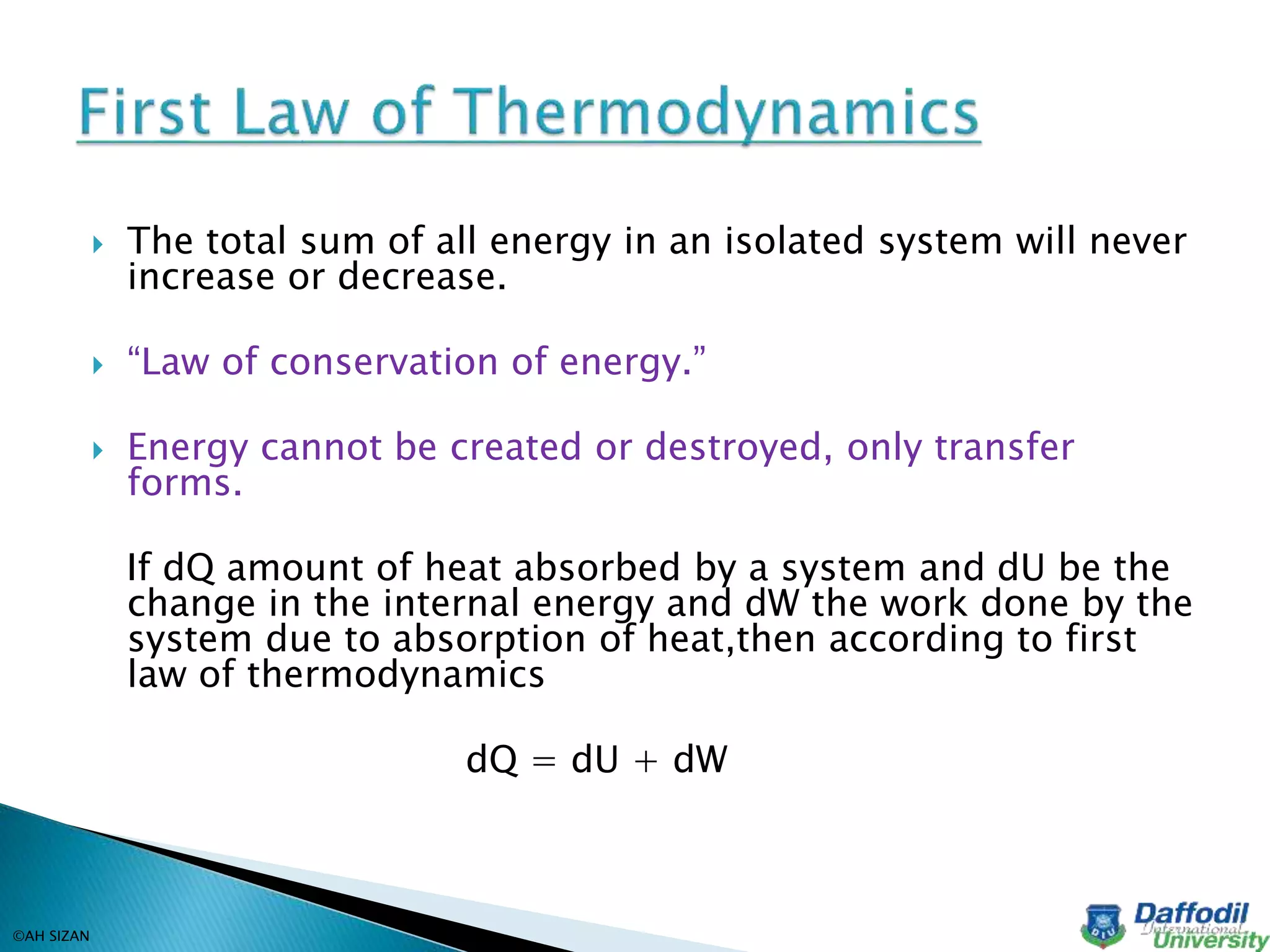
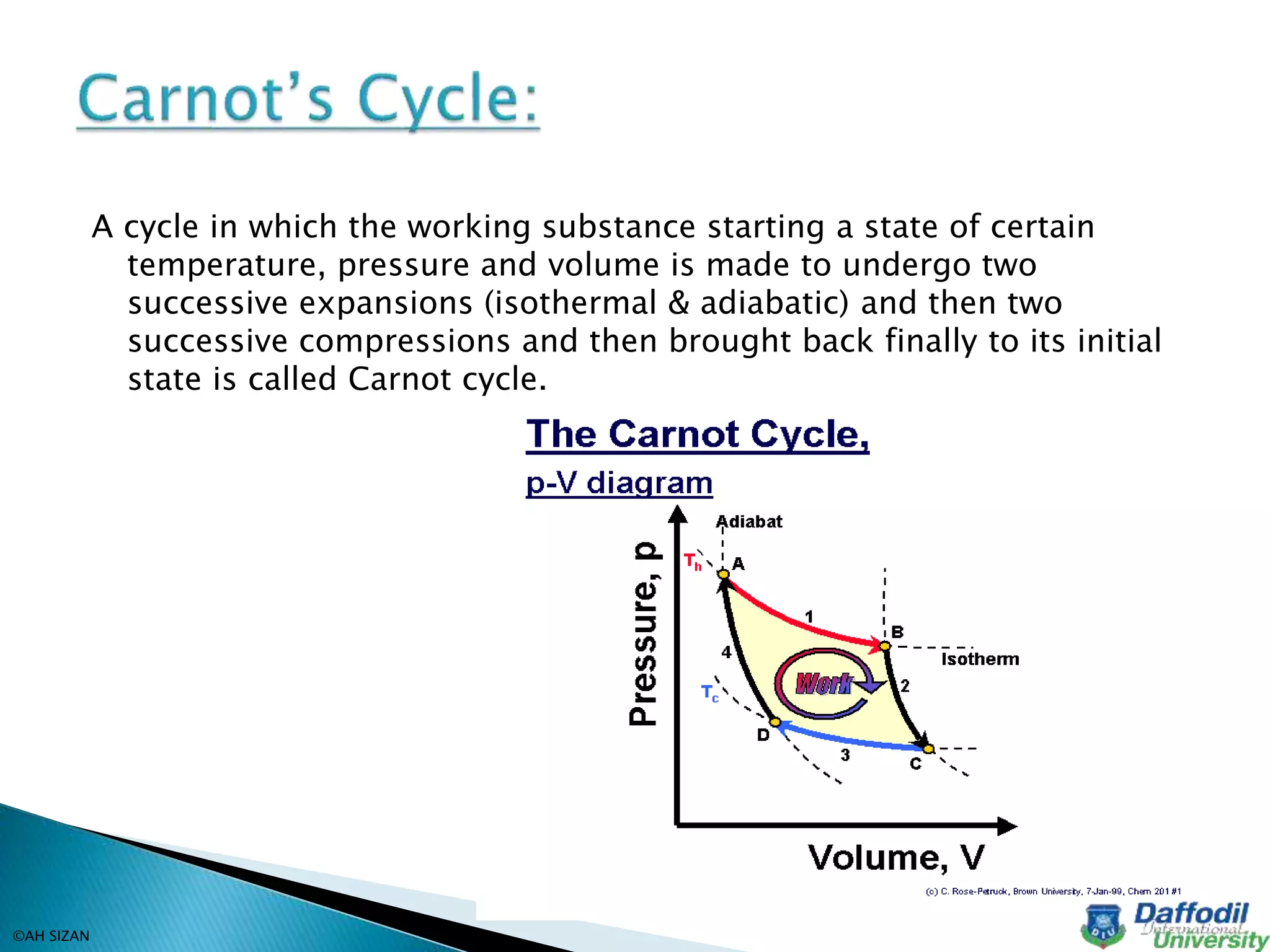
This document discusses key concepts in thermodynamics including heat, temperature, thermodynamics, the first law of thermodynamics, and reversible and irreversible processes. Thermodynamics is the branch of physics concerned with heat, temperature, and their relation to energy and work. Heat is a form of energy that flows from higher to lower temperature, and temperature determines the direction of heat flow. The first law of thermodynamics states that the total energy in an isolated system remains constant, as energy cannot be created or destroyed. Reversible processes can be retraced in the opposite direction, while irreversible processes cannot.