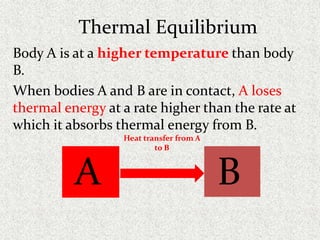
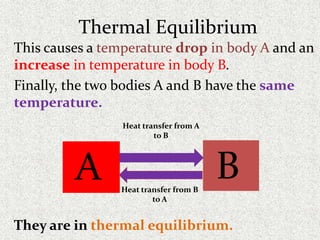
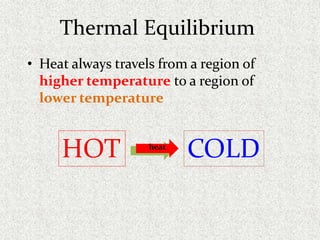
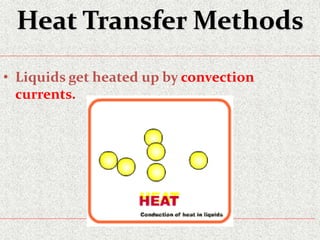
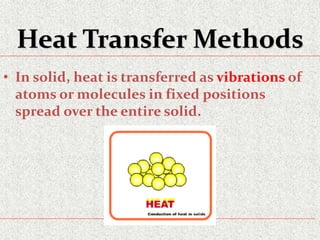
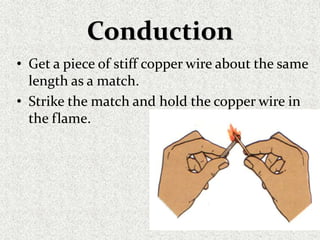
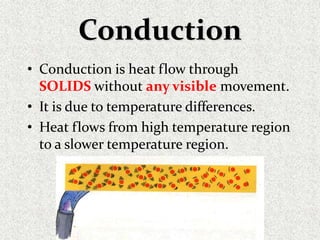
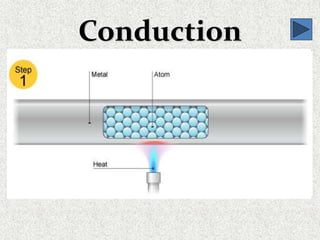
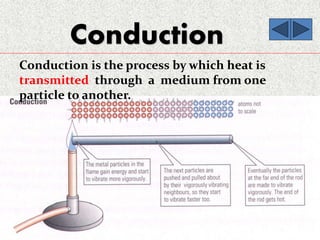
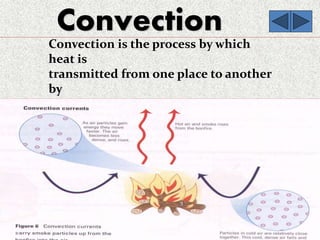
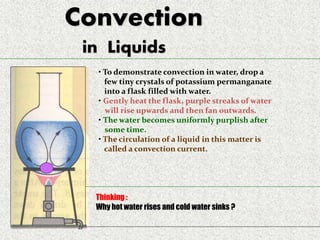
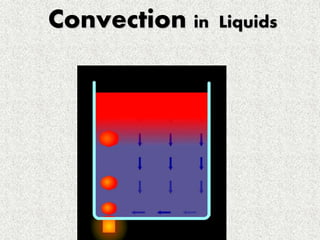
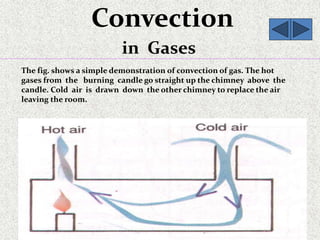
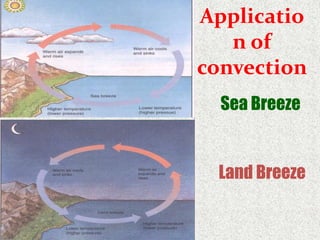
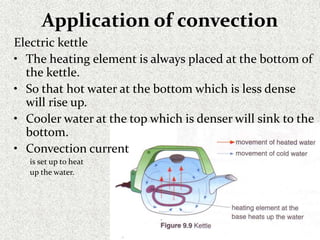
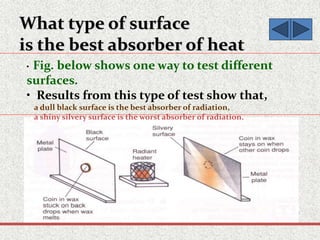
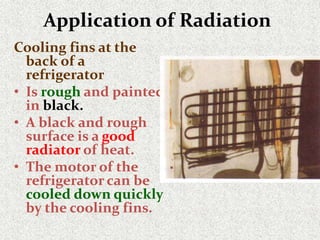
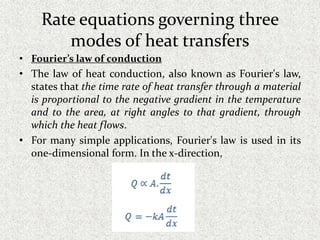
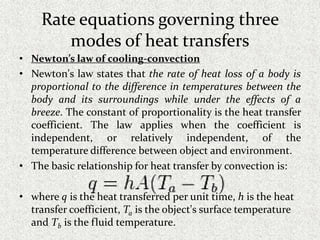
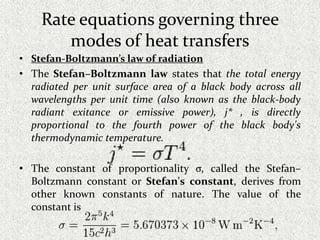
This document provides an introduction to heat transfer, including definitions, the three modes of heat transfer (conduction, convection, and radiation), and the fundamental laws governing heat transfer. It defines heat transfer as the movement of thermal energy from hotter to colder regions and discusses thermal equilibrium. The three modes of heat transfer - conduction through solids, convection through liquids and gases, and radiation through empty space - are described along with examples. The document also outlines the first and second laws of thermodynamics, Newton's laws of motion, and equations governing the rates of heat transfer via the three modes.