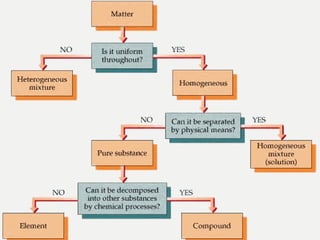
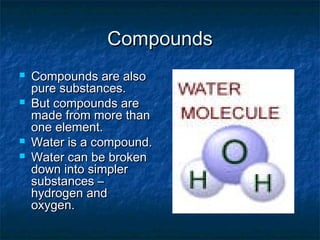
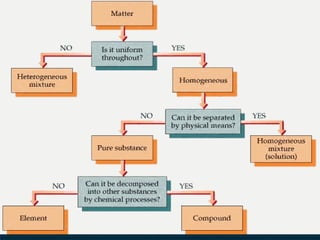
Matter can be classified into mixtures, elements, and compounds based on its composition. Mixtures consist of two or more substances that can be separated by physical means, whereas elements are pure substances that cannot be broken down further. Compounds are pure substances formed from two or more elements that can be separated by chemical means.