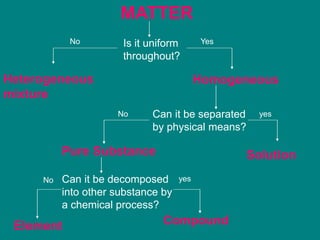
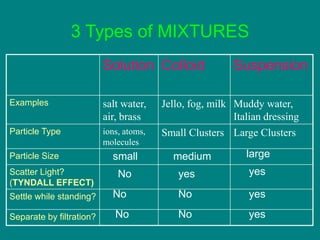
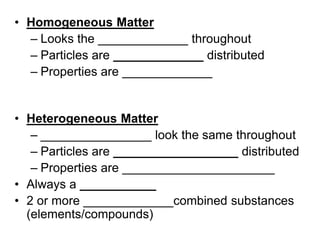
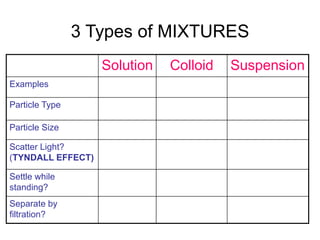
Matter can be classified as either pure substances or mixtures. Pure substances are either elements or compounds, while mixtures contain two or more substances physically combined. Mixtures can be either homogeneous, meaning the substances are evenly distributed, like solutions, or heterogeneous if the substances are not evenly distributed. Common types of mixtures include solutions, where particles are dissolved, colloids, where particles form small clusters, and suspensions, where large particles remain dispersed but can settle out of the mixture.