The document discusses labeling requirements for genetically modified organisms (GMOs) in the European Union. It states that foods and ingredients produced from GMOs must be labeled as such, including foods derived from GM crops like soy, corn, and rapeseed. Foods from animals fed GM feed do not require labeling. The labeling provides information for consumers and allows them to make informed choices. Traceability requirements ensure operators can identify suppliers and customers in the GM product supply chain.



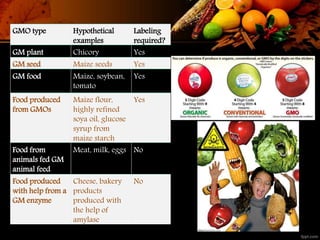

![ Labeling provides information for consumers and allows them to make an
informed choice.
In the case of pre-packaged GM food/feed products, the list of ingredients must
indicate "genetically modified" or "produced from genetically modified [name of
the organism]".
In the case of products without packaging these words must still be clearly
displayed in close proximity to the product (e.g a note on the supermarket
shelf).
These labeling requirements do not apply to GM food/feed products in a
proportion no higher than 0.9 percent of the food/feed ingredients considered
individually and if this presence is adventitious or technically unavoidable.](https://image.slidesharecdn.com/gmlabeling-210716104044/85/GM-labeling_Dr-Sonia-6-320.jpg)




