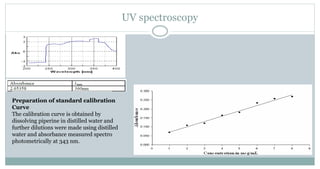
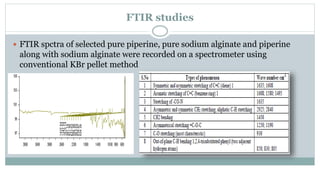
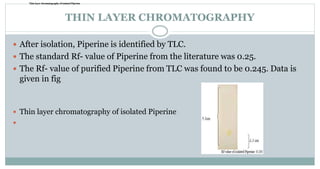
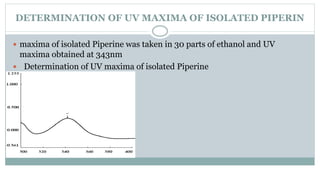
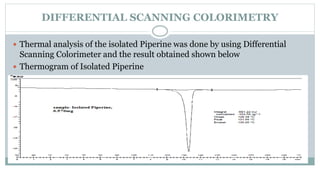
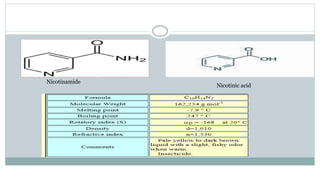
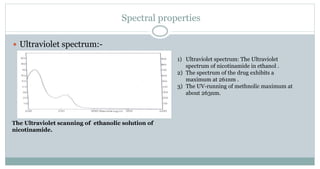
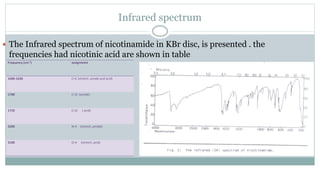
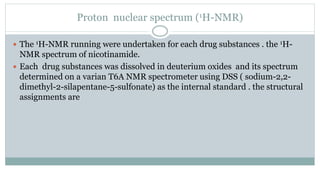
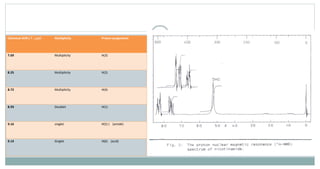
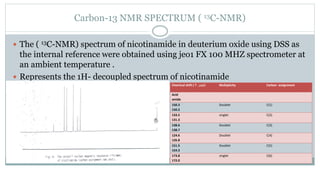
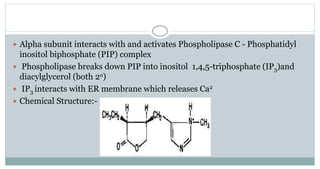
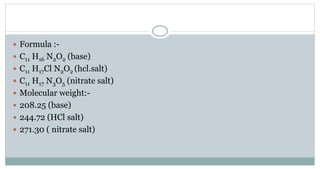
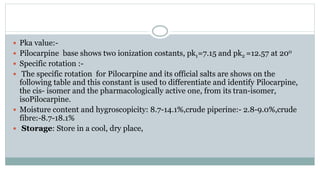
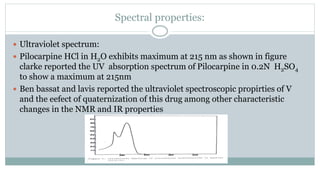
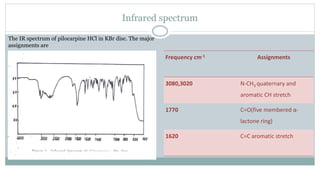
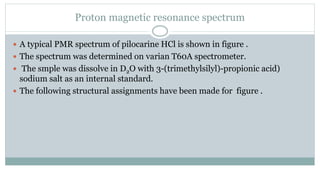
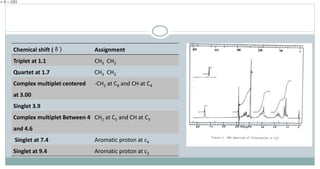
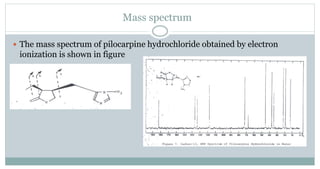
The document provides information on the structural elucidation of nicotine and pilocarpine. It discusses the isolation, chemical structure, nomenclature, physical properties, spectral properties, and identification tests of both compounds. Nicotine was isolated from tobacco leaves in 1828 and is a potent parasympathomimetic alkaloid. Pilocarpine is isolated from jaborandi leaves and binds to muscarinic receptors to activate various physiological responses. The document includes detailed 1H NMR, 13C NMR, IR, UV-Vis, and mass spectra to characterize the structure of nicotine and pilocarpine. Various chromatographic methods are also described for analytical testing.











![Nomenclature
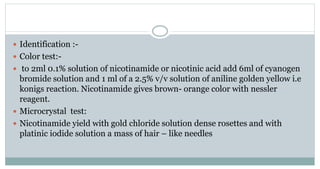
Chemical names:-
1) 1-[5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4- pentadienyl]piperidine
2) 5-(3,4-Methylenedioxyphenyl)-2,4-pentadienoyl-2- piperidine
3) Piperoylpiperidine
4)Bioperine
structure:-](https://image.slidesharecdn.com/structuralelucidation-150311053724-conversion-gate01/85/Structural-elucidation-32-320.jpg)



![High Performance Liquid Chromatography (HPLC) Analysis
HPLC Analysis of extract
File name: H piperine 50 ppm
MP: water: MeOH [30:70]
Column: HiQ Sil C18W
Flow rate: 1 ml/min](https://image.slidesharecdn.com/structuralelucidation-150311053724-conversion-gate01/85/Structural-elucidation-36-320.jpg)