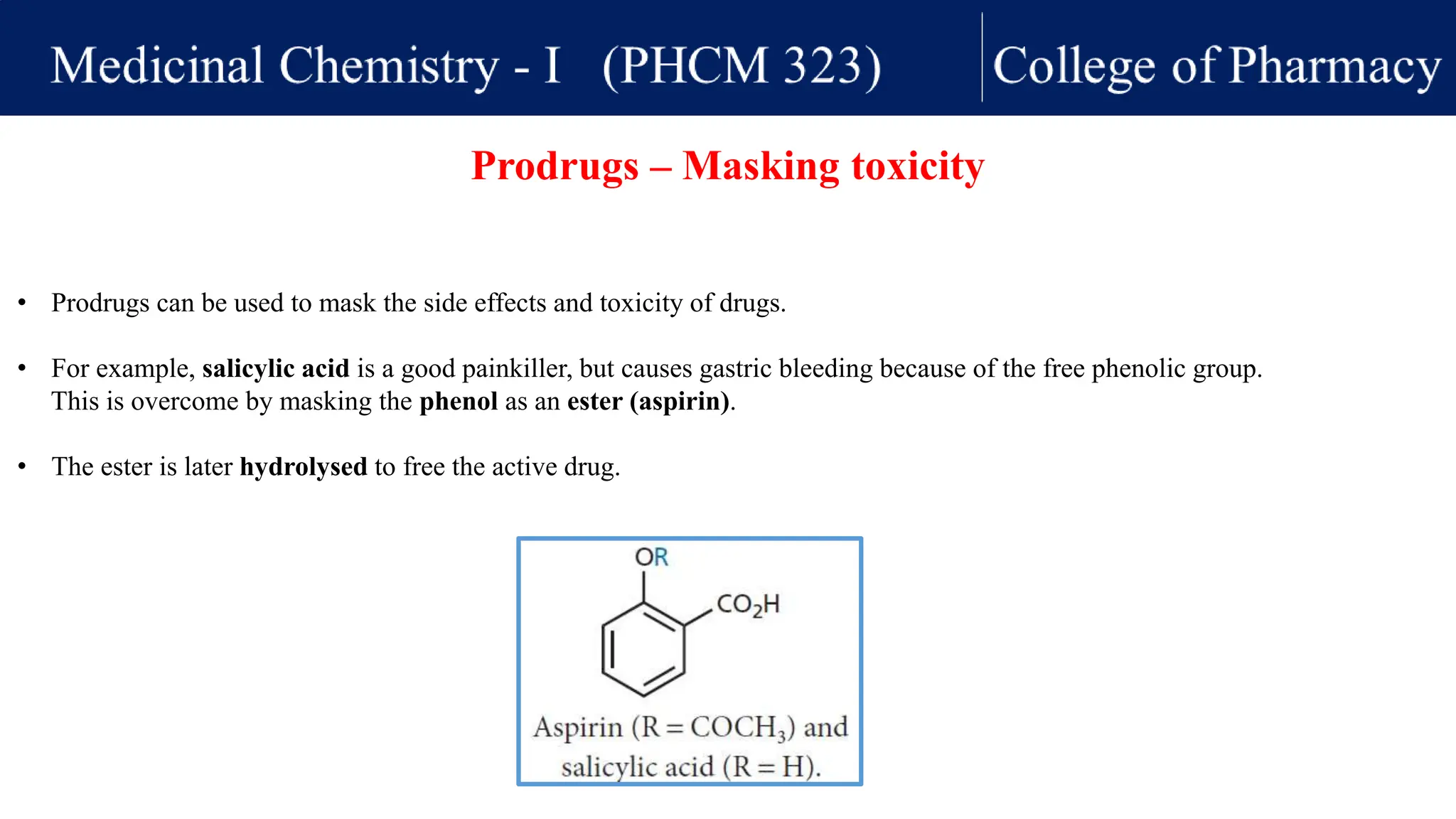
This lecture discusses structure-activity relationships (SAR) and prodrugs. For SAR, analogs of lead compounds are synthesized with small modifications to identify important structural features for biological activity. Examining alcohol, phenol, aromatic, alkene, ketone, amine, carboxylic acid analogs can provide insights. The pharmacophore summarizes critical structural features. Prodrugs are inactive compounds converted in vivo to active drugs, addressing issues like permeability, toxicity, or sustained release. They include ester prodrugs for permeability and azathioprine for prolonged activity. Masking moieties can also reduce toxicity as in aspirin. Some prodrugs selectively release drugs at disease sites, like met