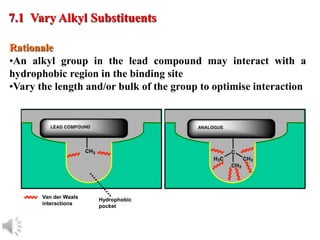
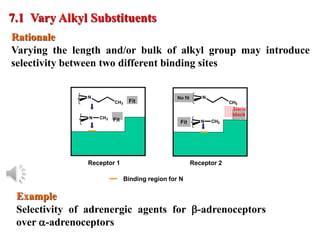
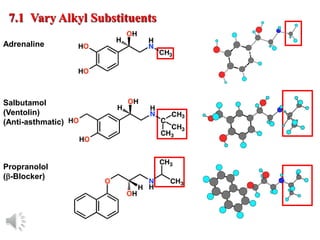
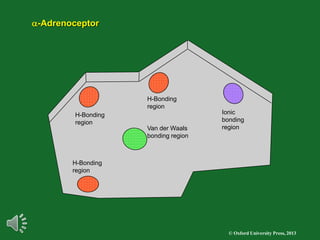
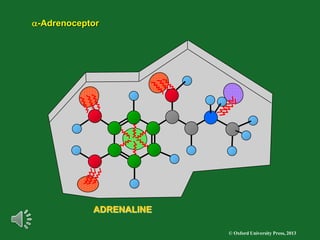
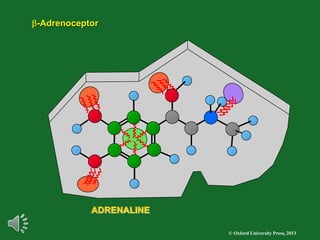
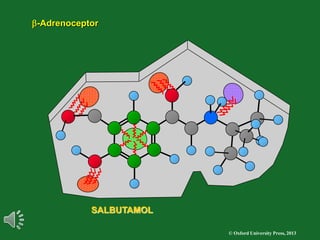
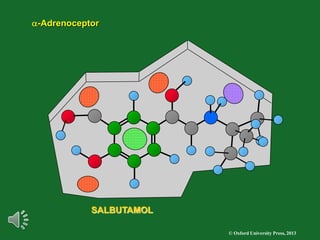
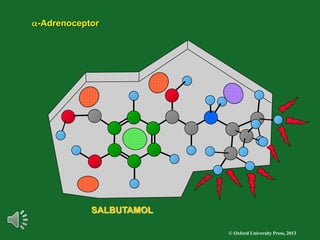
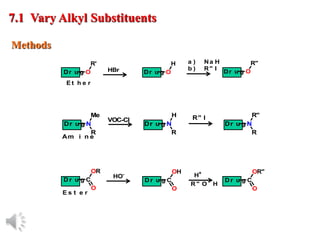
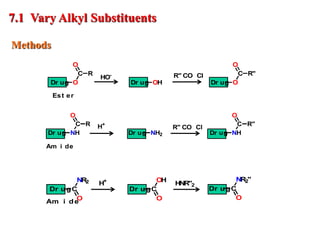
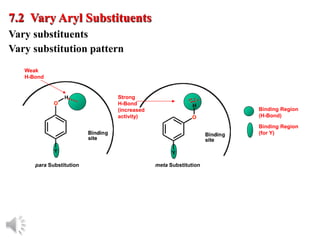
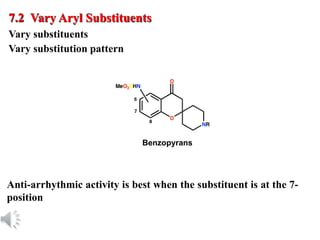
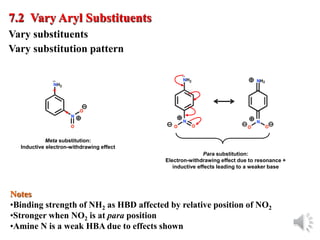
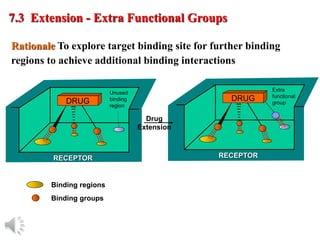
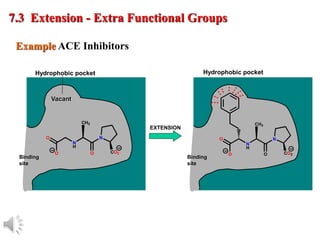
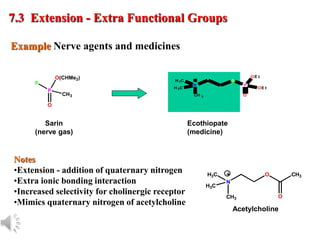
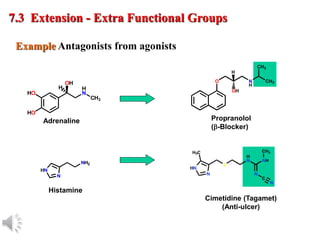
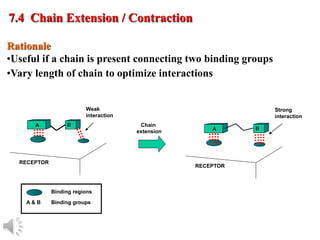
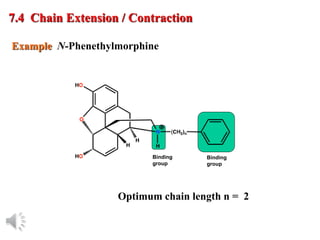
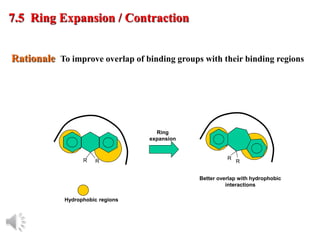
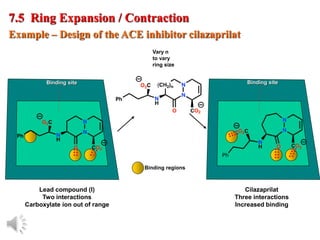
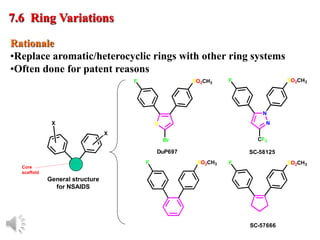
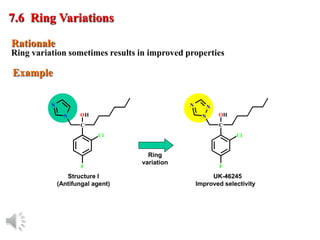
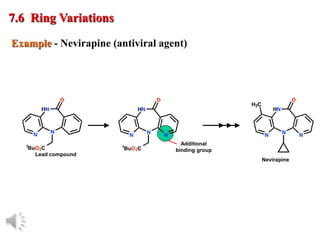
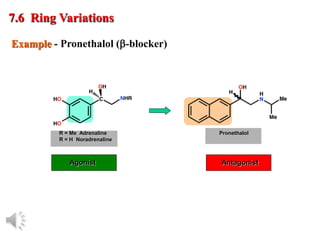
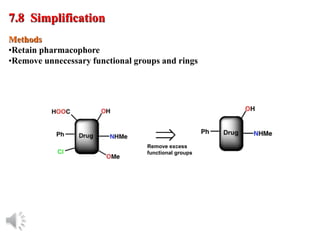
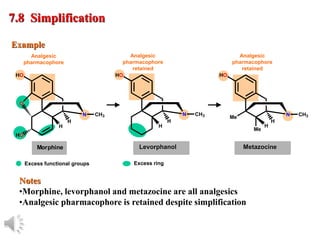
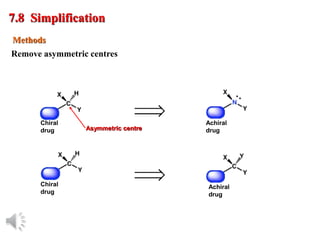
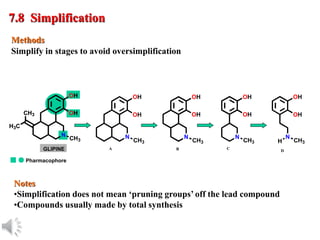
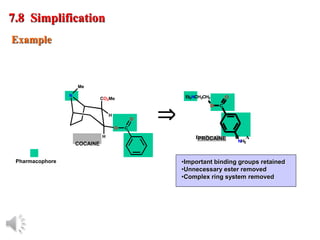
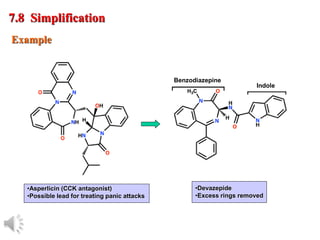
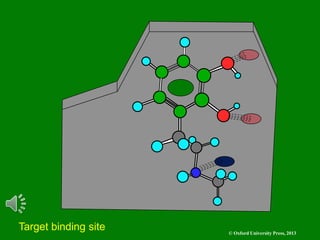
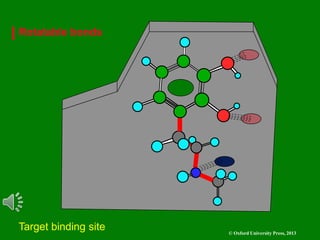
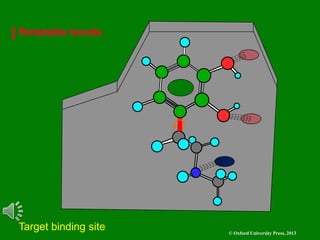
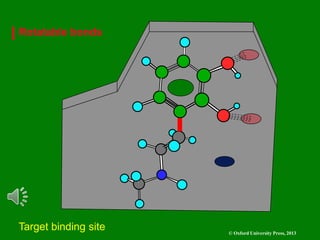
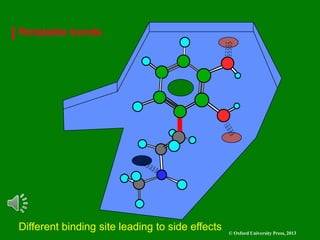
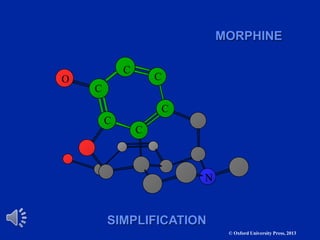
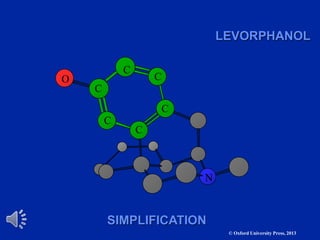
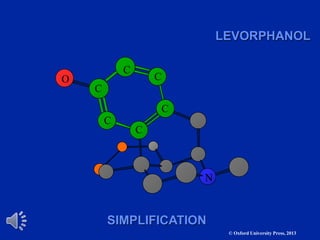
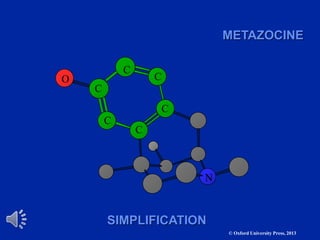
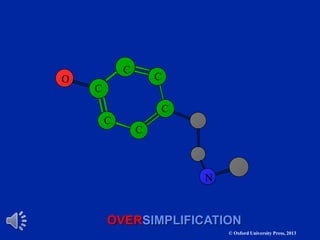
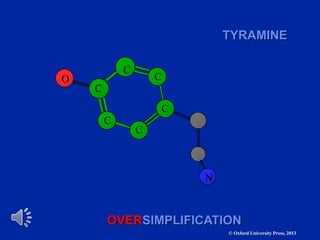
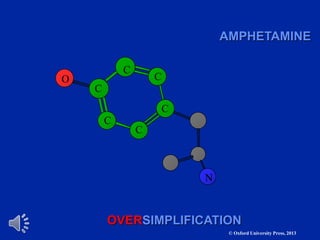
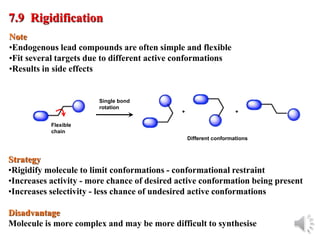
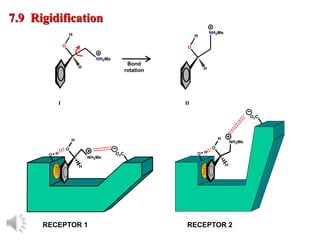
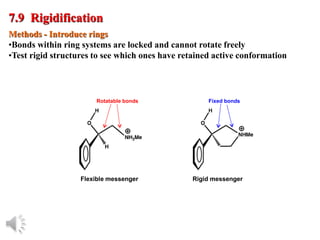
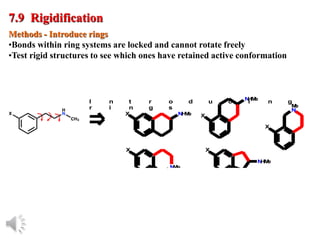
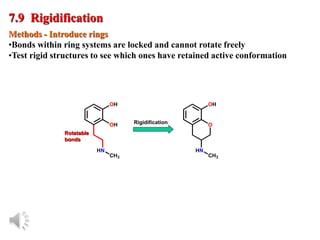
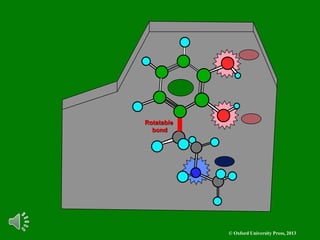
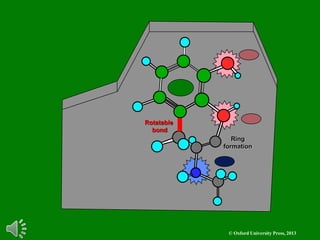
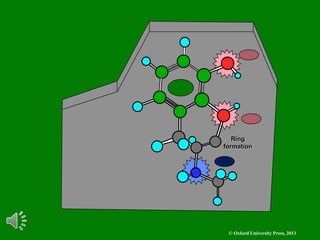
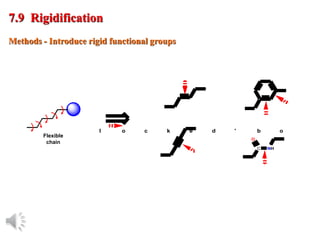
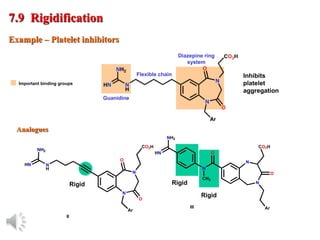
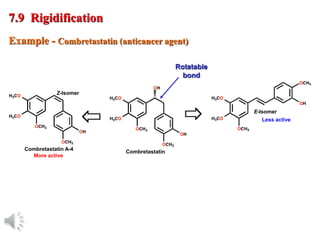
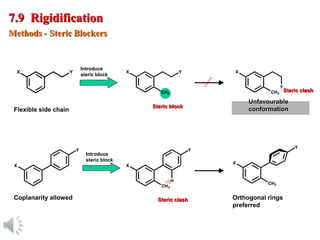
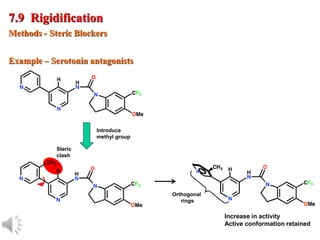
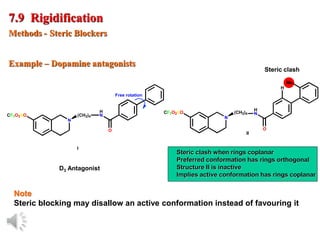
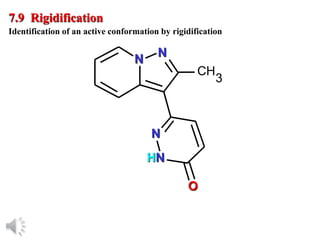
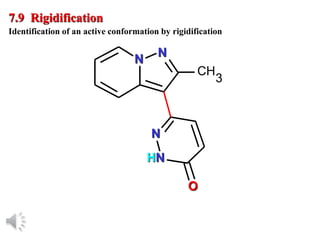
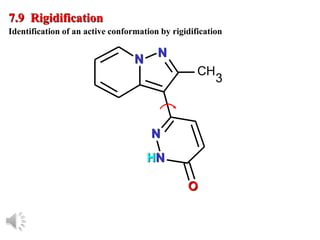
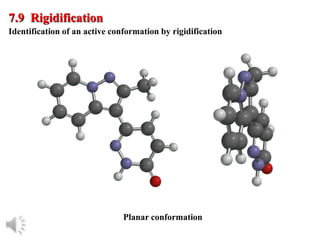
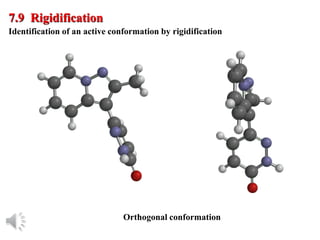
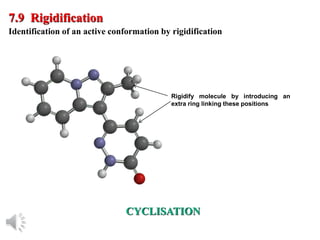
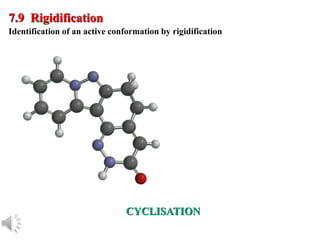
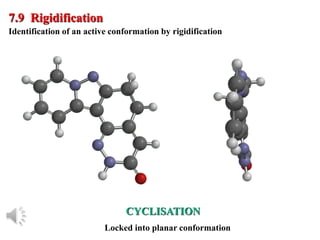
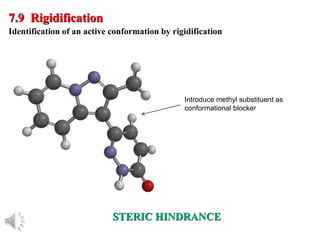
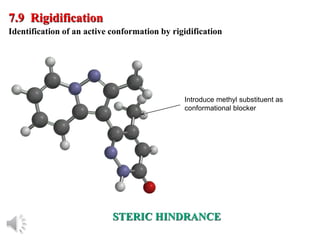
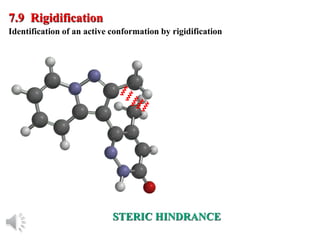
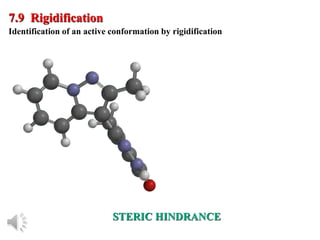
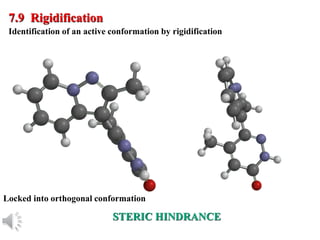
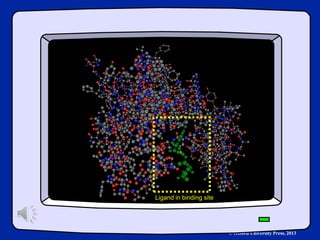
The document outlines the stages of drug design and development, including identifying target diseases, establishing testing procedures, and optimizing target interactions. It discusses strategies for optimizing drug properties through variations in alkyl and aryl substituents, extensions, ring modifications, and simplification techniques. Additionally, it emphasizes the importance of maintaining selectivity and minimizing side effects during the drug design process.