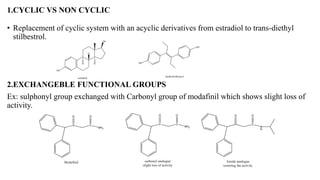
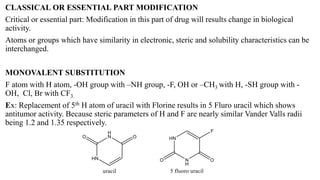
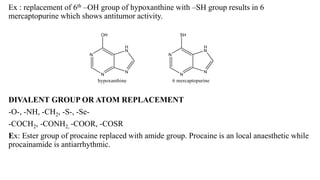
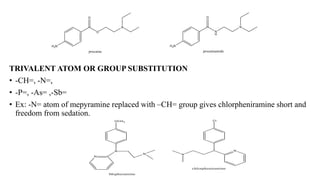
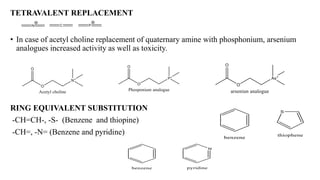
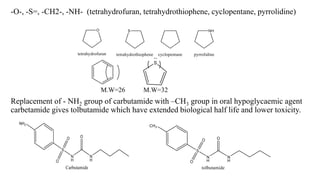
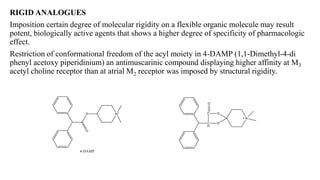
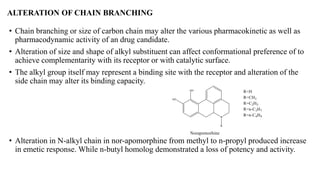
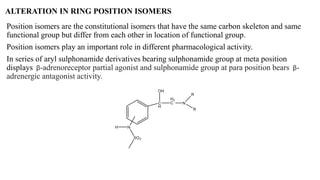
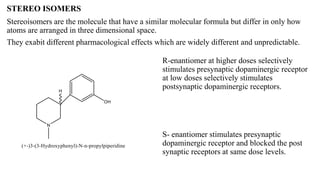
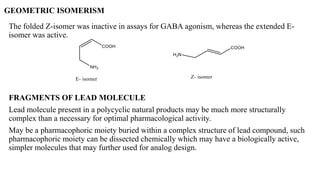
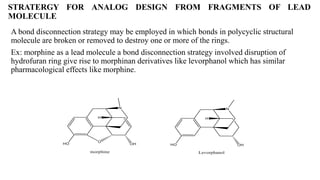
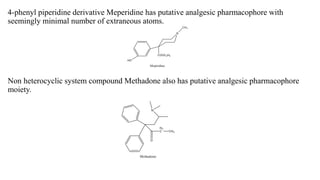
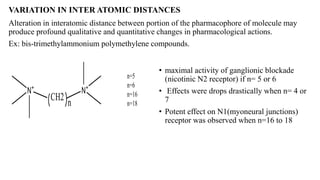
This document summarizes strategies for analog design of lead compounds in drug discovery. It discusses various types of modifications that can be made including bioisosteric replacements, rigid analogs, alterations of chain branching, changes in ring size or position, use of fragments of lead molecules, and variations in interatomic distances. Examples are provided to illustrate how each type of modification can impact pharmacological activity. The overall goal of analog design is to develop new compounds with similar or improved biological and chemical properties as the lead molecule.